Figure 3.
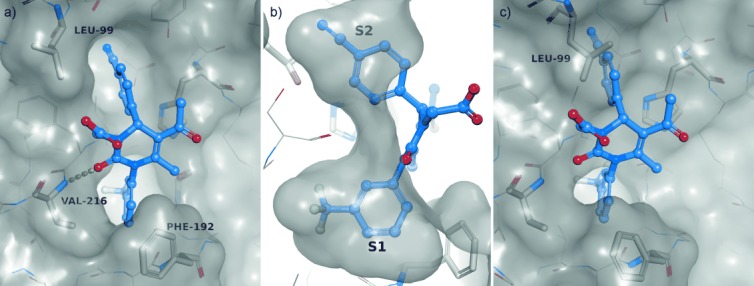
Induced-fit binding mode. Protease (HNE) residues are shown in stick representation (white) with transparent Connolly-like surface.[28], [29] Ligand 19 (purple) is shown in ball-and-stick model (oxygen: red, nitrogen: blue, fluorine: cyan); hydrogen bonds are depicted as broken yellow lines. a) Structure of HNE in complex with 19. Ligand 19 interacts with HNE by a hydrogen bond (3.1 Å) formed between the C2 carbonyl oxygen atom of the central pyrimidine ring and the Val216 backbone amide of HNE. b) Binding to the S1 and S2 subsites is governed by exact protein–ligand shape complementarity of the northern and southern phenyl spheres of 19. c) Binding conformation of 19 overlaid on the binding site of apo-HNE. In the apo structure the S2 subsite next to Leu99 is not large enough to accommodate the northern para-cyanophenyl ring. Binding is only possible through an induced-fit mechanism, in which Leu99B is rotated toward the bulk solvent, thus expanding the lipophilic S2 pocket.
