Scheme 2.
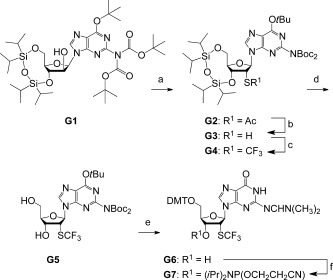
Synthesis of 2′-SCF3 guanosine phosphoramidite G7. Starting compound G1 was obtained according to ref. [27]. Reaction conditions: a) i. 1.5 equiv F3CSO2Cl, 3.0 equiv DMAP, in CH2Cl2, 0 °C, 20 min; ii. 1.5 equiv CH3COS−K+, 1.5 equiv 18-crown-6, 1.5 equiv EtN(iPr)2 in toluene, 16 h, 45 °C, 82 %; b) 1.6 M MeNH2, in EtOH/CH2Cl2 (23:1), 0 °C, 25 min, 85 %; c) 1.2 equiv 3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole, CH2Cl2, −78 °C to RT, 16 h, 73 %; d) 1 M TBAF, in THF, RT, 3.5 h, 81 %; e) i. CF3COOH/CH2Cl2 (1:7), RT, 2.5 h; ii. 3.0 equiv (H3CO)2CHN(CH3)2, in CH3OH, reflux, 6 h; iii. 1.1 equiv DMT-Cl, 0.1 equiv DMAP, in pyridine, RT, 18 h, 48 %; f) 1.5 equiv 2-cyanoethyl N,N-diisopropylchlorophosphoramidite, 10 equiv CH3CH2N(CH3)2, CH2Cl2, RT, 3 h, 72 %.
