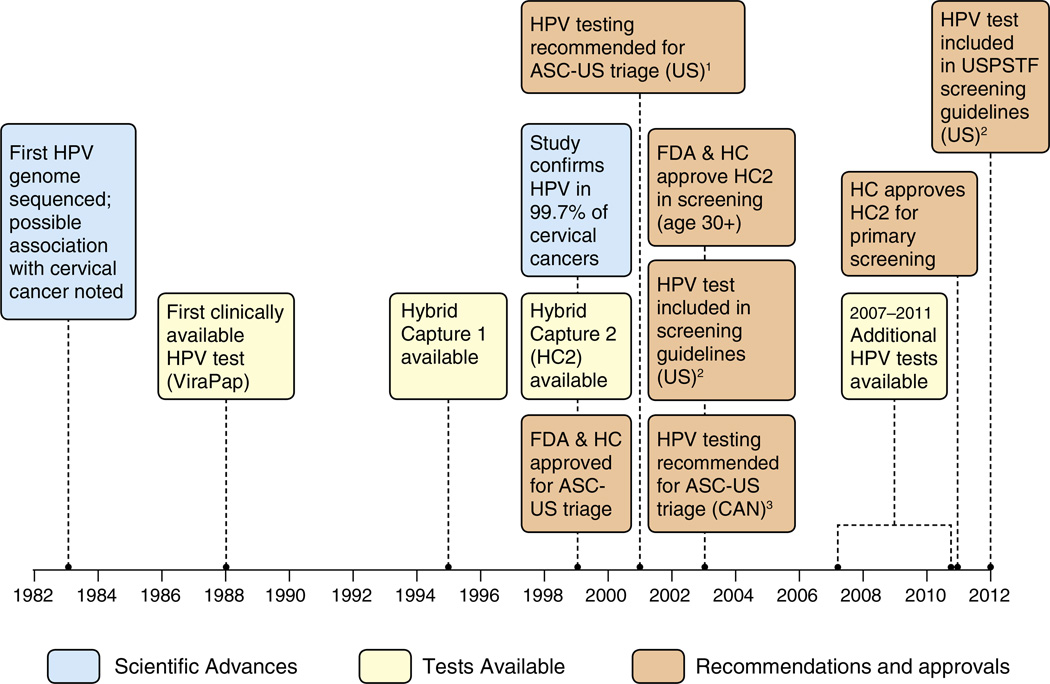
Fig. 1.
Evolution of HPV testing in Canada and the United States. Footnotes: US=United States. CAN=Canada. FDA=US Food and Drug administration. HC=Health Canada. USPSTF=US Preventive Services Task Force. ASC-US=Atypical squamous cells of undetermined significance. HPV=Human Papillomavirus. HPV testing was first recommended for triage of ASC-US lesions by the American Society for Colposcopy and Cervical Pathology in 2001. The American Cancer Society and American College of Obstetricians and Gynecologists recommended HPV tests as an option for screening women 30 years and older starting in 2003. The US Preventive service Task Force made a similar recommendation in 2012. The Pan-Canadian Forum on Cervical Cancer Prevention and Control first recommended HPV testing for triage of ASCUS lesions in Canada in 2003. The Society of Obstetricians and Gynecologists of Canada made a similar recommendation in 2007. SOURCES: Cox, JT. History of the use of HPV testing in cervical screening and in the management of abnormal cervical screening results. Journal of Clinical Virology 2009;45:S3–S12. Centers for Disease Control and Prevention. Cervical Cancer Screening Guidelines for Average-Risk Women. Available at http://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed November 6, 2012. FDA. U.S. Food and Drug Administration website. Available at www.fda.gov. Accessed November 6, 2012.