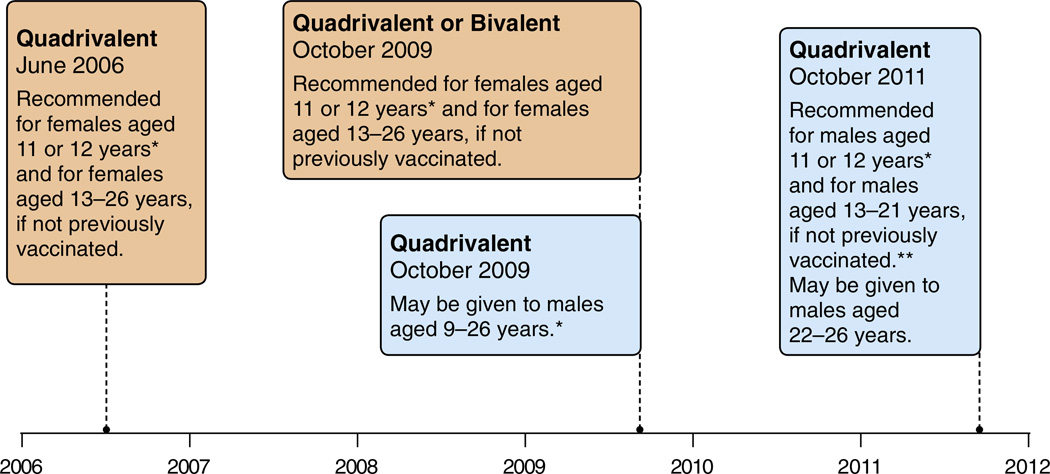
Fig. 2.
United States: evolution of recommendations for HPV vaccination from the Advisory Committee on Immunization Practice (ACIP). Footnotes: Quadrivalent (HPV 6,11,16,18) vaccine; Bivalent (HPV 16,18) vaccine. *Can be given starting at 9 years of age;. **For MSM and immunocompromised males, quadrivalent HPV vaccine through 26 years of age. SOURCES: Centers for Disease Control and Prevention. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm. Rep. 56, 1–24. Centers for Disease Control Prevention, 2010. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly Rep. 59, 626–629. Centers for Disease Control and Prevention, 2011. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb. Mortal. Wkly Rep. 60, 1705–1708.