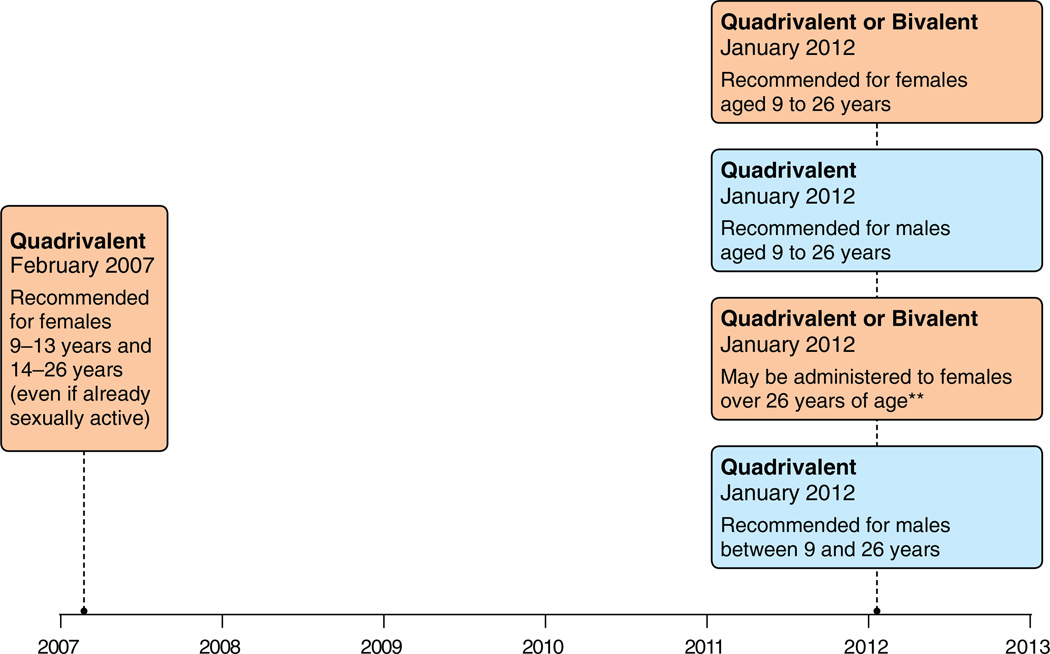
Fig. 3.
Canada: Evolution of Recommendations for HPV Vaccination from the National Advisory Committee on Immunization (NACI)*. Footnotes: Quadrivalent (HPV 6,11,16,18) vaccine; Bivalent (HPV 16,18) vaccine. *Recommendations are adapted by provincial and territorial programs. *Grade A for quadrivalent and Grade B for bivalent. National Advisory Committee on Immunization (NACI). Statement on human papillomavirus vaccine. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2007;33 ACS-2: 1–31. Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Update on Human Papillomavirus (HPV) Can Commun Dis Rep 2012;38 ACS-1:1–62. Note: NACI makes recommendations, but it is up to the various provincial and territorial authorities to adapt them to their specific vaccine programs.