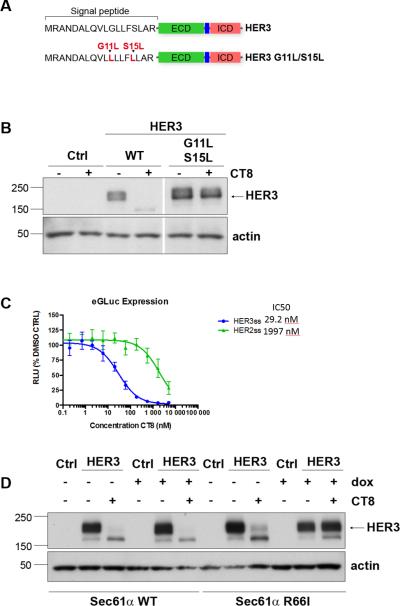
Figure 3. HER3 suppression by CT8 is mediated through the signal peptide.
A. Schematic representation showing the site of the engineered mutations. B. HEK-293 cells were transiently transfected to express either WT HER3 or the indicated HER3 mutant shown in the schematic. Following transfection, cells were treated with 500nM CT8 for 24 hours and HER3 expression in cell lysates assayed by western blotting. The lanes were run on the same gel but were noncontiguous. C. HEK-293T cells transfected with plasmids encoding the mature domain of enhanced Gaussia luciferase (eGluc) fused to the signal sequence of HER2 or HER3 were incubated in doxycycline (1 μg/mL, to induce expression) and increasing concentrations of CT8 for 24 hours, after which luminescence was measured. D. HEK-293 cells with the tet-inducible induction of expression of wildtype Sec61α or the CT8-resistant R66I mutant Sec61α were incubated in doxycycline (1 μg/mL, to induce expression). The following day, cells were transfected with an empty vector or a HER3-expressing vector, and 4 hours later placed in media containing DMSO or 500nM CT8. After 24 hours of treatment, cell lysates were harvested and assayed for expression of HER3 by western blotting. Molecular mass standards (kDa) are indicated on the left side of the figure.