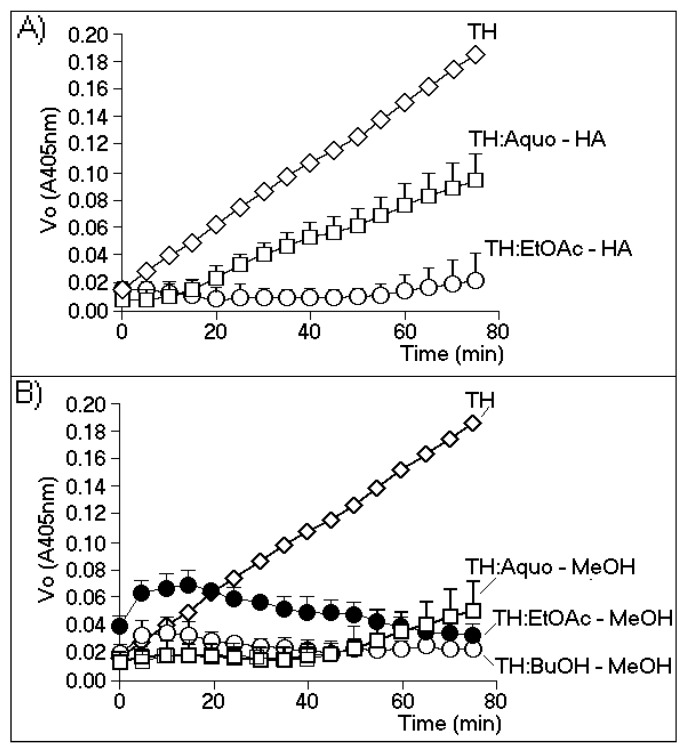
Figure 2.
The chromogenic substrate for thrombin is specifically cleaved by thrombin at a slow rate. The biochemical reaction was b-Ala-Gly-Arg-p-nitroanilide → b-Ala-Gly-Arg + p-nitroanilide, which was measured at 405 nm. The method used for this evaluation was based on [18] and the manufacturer’s instructions. All enzymatic assays were performed using a SPECTRA MAX (Molecular Devices, Sunnyvale, CA, USA), with n = 12. (A) The effects of the aqueous phase (Aquo-HA) and ethyl acetate (EtOAc-HA) phase of the hydroalcoholic extract; (B) the inhibitory effects of the aqueous (Aquo-MeOH), ethyl acetate (EtOAc-MeOH) and butanolic (BuOH-MeOH) phases of the methanolic extract.