Abstract
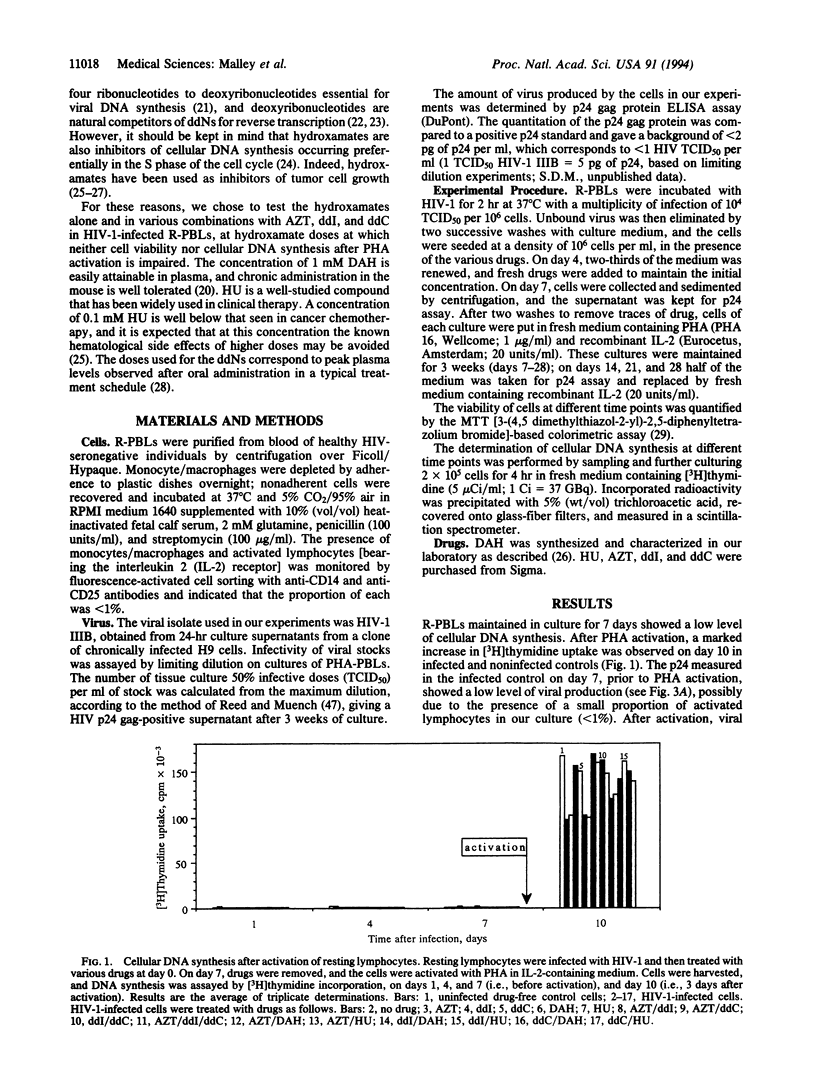
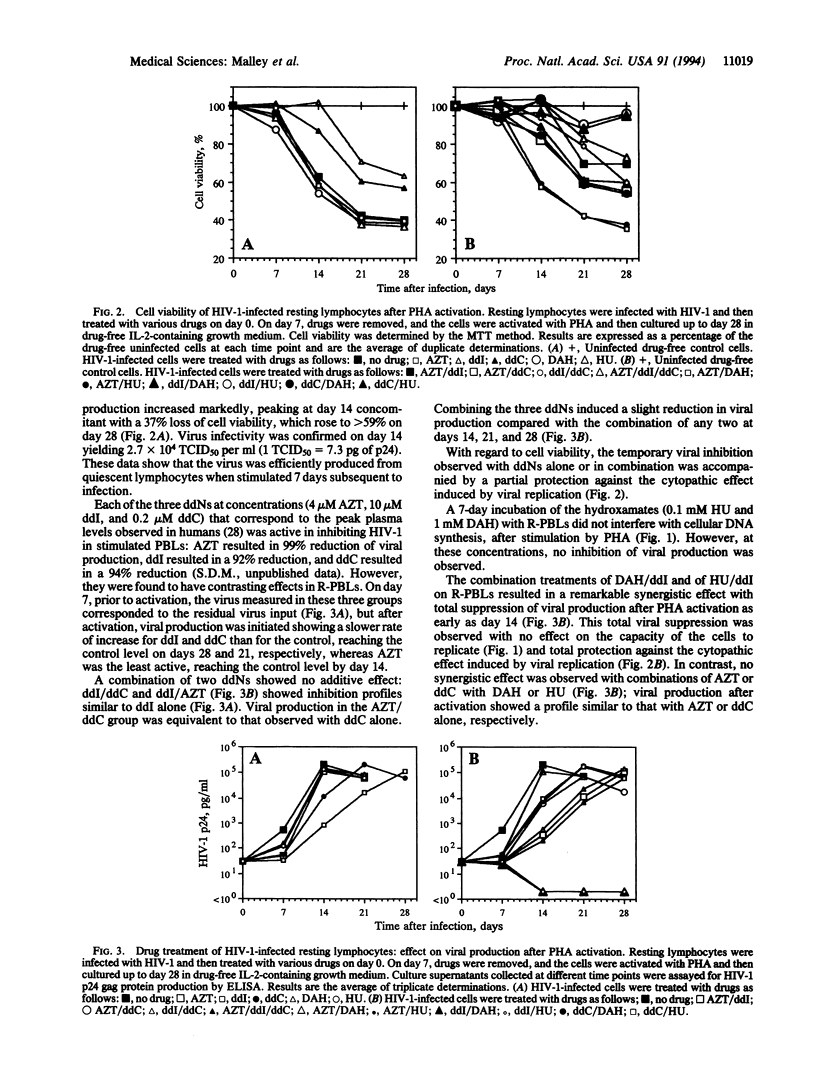
The cellular models generally used in the in vitro evaluation of anti-human immunodeficiency virus compounds are dividing cells. A model constituted by resting lymphocytes may more accurately reflect a drug's future efficacy in humans, since viral DNA synthesis is known to take place in quiescent cells, creating a reservoir of infected cells awaiting activation to complete their viral replication cycle and to produce infectious virions. We report here the activity of 3'-azido-3'-deoxythymidine, 2',3'-dideoxyinosine, 2',3'-dideoxycytidine, and two hydroxamates, D-aspartic acid beta-hydroxamate and hydroxycarbamate (hydroxyurea), alone and in various combinations, in an in vitro model based on resting lymphocytes. In our model, resting peripheral blood lymphocytes were infected with human immunodeficiency virus type 1 and treated with drugs for 7 days, at which time drugs were removed and the cells were activated by phytohemagglutinin. We show that under these conditions 3'-azido-3'-deoxythymidine, 2',3'-dideoxyinosine, and 2',3'-dideoxycytidine, alone or in combination, neither fully inhibit viral production nor protect lymphocytes from the cytopathic effect of viral replication, at concentrations corresponding to the peak plasma levels observed in a typical treatment schedule in humans. In contrast, we report the synergistic effect of treatment by each hydroxamate with 2',3'-dideoxyinosine of infected resting lymphocytes, resulting in the total suppression of viral production, total protection against the cytopathic effect induced by viral replication, and no effect on the ability of the cells to replicate in this cell culture system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. O., Wilkes S. H., Bayliss M. E., Prescott J. M. Hydroxamates and aliphatic boronic acids: marker inhibitors for aminopeptidase. Biochemistry. 1983 Apr 26;22(9):2098–2103. doi: 10.1021/bi00278a009. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Stanwick T. L., Dempsey M. P., Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991 Oct 18;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K. J., Hammer S. M. Antiretroviral therapy: strategies beyond single-agent reverse transcriptase inhibition. Antimicrob Agents Chemother. 1992 Mar;36(3):509–520. doi: 10.1128/aac.36.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. F., 2nd, Hostomska Z., Hostomsky Z., Jordan S. R., Matthews D. A. Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase. Science. 1991 Apr 5;252(5002):88–95. doi: 10.1126/science.1707186. [DOI] [PubMed] [Google Scholar]
- De Clercq E. HIV inhibitors targeted at the reverse transcriptase. AIDS Res Hum Retroviruses. 1992 Feb;8(2):119–134. doi: 10.1089/aid.1992.8.119. [DOI] [PubMed] [Google Scholar]
- Donehower R. C. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992 Jun;19(3 Suppl 9):11–19. [PubMed] [Google Scholar]
- Elford H. L., Freese M., Passamani E., Morris H. P. Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem. 1970 Oct 25;245(20):5228–5233. [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Jildevik I., Skog S., Thelander L., Tribukait B. Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J Biol Chem. 1985 Aug 5;260(16):9114–9116. [PubMed] [Google Scholar]
- Eriksson S., Martin D. W., Jr Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem. 1981 Sep 25;256(18):9436–9440. [PubMed] [Google Scholar]
- Gao W. Y., Cara A., Gallo R. C., Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Shirasaka T., Johns D. G., Broder S., Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Invest. 1993 May;91(5):2326–2333. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel J., Rassart E., Jolicoeur P. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology. 1981 Apr 15;110(1):202–207. doi: 10.1016/0042-6822(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., D'Aquila R. T. Therapy for human immunodeficiency virus infection. N Engl J Med. 1993 Jun 10;328(23):1686–1695. doi: 10.1056/NEJM199306103282307. [DOI] [PubMed] [Google Scholar]
- Holmes M. A., Matthews B. W. Binding of hydroxamic acid inhibitors to crystalline thermolysin suggests a pentacoordinate zinc intermediate in catalysis. Biochemistry. 1981 Nov 24;20(24):6912–6920. doi: 10.1021/bi00527a026. [DOI] [PubMed] [Google Scholar]
- Huang P., Farquhar D., Plunkett W. Selective action of 3'-azido-3'-deoxythymidine 5'-triphosphate on viral reverse transcriptases and human DNA polymerases. J Biol Chem. 1990 Jul 15;265(20):11914–11918. [PubMed] [Google Scholar]
- Mayers D. L., McCutchan F. E., Sanders-Buell E. E., Merritt L. I., Dilworth S., Fowler A. K., Marks C. A., Ruiz N. M., Richman D. D., Roberts C. R. Characterization of HIV isolates arising after prolonged zidovudine therapy. J Acquir Immune Defic Syndr. 1992;5(8):749–759. [PubMed] [Google Scholar]
- Merigan T. C., Skowron G., Bozzette S. A., Richman D., Uttamchandani R., Fischl M., Schooley R., Hirsch M., Soo W., Pettinelli C. Circulating p24 antigen levels and responses to dideoxycytidine in human immunodeficiency virus (HIV) infections. A phase I and II study. Ann Intern Med. 1989 Feb 1;110(3):189–194. doi: 10.7326/0003-4819-110-3-189. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Jarrett R. F., Matsukura M., Di Marzo Veronese F., DeVico A. L., Sarngadharan M. G., Johns D. G., Reitz M. S., Broder S. Long-term inhibition of human T-lymphotropic virus type III/lymphadenopathy-associated virus (human immunodeficiency virus) DNA synthesis and RNA expression in T cells protected by 2',3'-dideoxynucleosides in vitro. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2033–2037. doi: 10.1073/pnas.84.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Yarchoan R., Broder S. Molecular targets for AIDS therapy. Science. 1990 Sep 28;249(4976):1533–1544. doi: 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Mead J. R., Feorino P. M. Insights into HIV chemotherapy. AIDS Res Hum Retroviruses. 1992 Jun;8(6):963–990. doi: 10.1089/aid.1992.8.963. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Sommadossi J. P., Saalmann V., Cannon D. L., Xie M. Y., Hart G. C., Smith G. A., Hahn E. F. Activities of 3'-azido-3'-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1990 Jun;34(6):1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T., Yarchoan R., O'Brien M. C., Husson R. N., Anderson B. D., Kojima E., Shimada T., Broder S., Mitsuya H. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):562–566. doi: 10.1073/pnas.90.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Henderson L. E., Chance M. R., Bess J. W., Jr, South T. L., Blake P. R., Sagi I., Perez-Alvarado G., Sowder R. C., 3rd, Hare D. R. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992 May;1(5):563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasset N., Hamedi-Sangsari F., Tournaire R., Navarro C., Malley S., Goetsch L., Grange J., Vila J. Anti-tumoral activity of L and D isomers of aspartic acid beta-hydroxamate on L5178Y leukemia. Int J Cancer. 1991 Sep 30;49(3):421–424. doi: 10.1002/ijc.2910490319. [DOI] [PubMed] [Google Scholar]
- Tournaire R., Malley S., Hamedi-Sangsari F., Thomasset N., Grange J., Dore J. F., Vila J. Therapeutic effects of D-aspartic acid beta-hydroxamate (DAH) on Friend erythroleukemia. Int J Cancer. 1994 Aug 1;58(3):420–425. doi: 10.1002/ijc.2910580319. [DOI] [PubMed] [Google Scholar]
- Törnevik Y., Jacobsson B., Britton S., Eriksson S. Intracellular metabolism of 3'-azidothymidine in isolated human peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1991 Sep;7(9):751–759. doi: 10.1089/aid.1991.7.751. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Vila J., Thomasset N., Navarro C., Doré J. F. In vitro and in vivo anti-tumor activity of L-glutamic acid gamma-monohydroxamate against L1210 leukemia and B16 melanoma. Int J Cancer. 1990 Apr 15;45(4):737–743. doi: 10.1002/ijc.2910450428. [DOI] [PubMed] [Google Scholar]
- Yarbro J. W. Further studies on the mechanism of action of hydroxyurea. Cancer Res. 1968 Jun;28(6):1082–1087. [PubMed] [Google Scholar]
- Yarbro J. W. Mechanism of action of hydroxyurea. Semin Oncol. 1992 Jun;19(3 Suppl 9):1–10. [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Myers C. E., Broder S. Clinical pharmacology of 3'-azido-2',3'-dideoxythymidine (zidovudine) and related dideoxynucleosides. N Engl J Med. 1989 Sep 14;321(11):726–738. doi: 10.1056/NEJM198909143211106. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Perno C. F., Thomas R. V., Klecker R. W., Allain J. P., Wills R. J., McAtee N., Fischl M. A., Dubinsky R., McNeely M. C. Phase I studies of 2',3'-dideoxycytidine in severe human immunodeficiency virus infection as a single agent and alternating with zidovudine (AZT). Lancet. 1988 Jan 16;1(8577):76–81. doi: 10.1016/s0140-6736(88)90283-8. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Cann A. J., Lugo J. P., Chen I. S. HIV-1 production from infected peripheral blood T cells after HTLV-I induced mitogenic stimulation. Science. 1988 May 20;240(4855):1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


