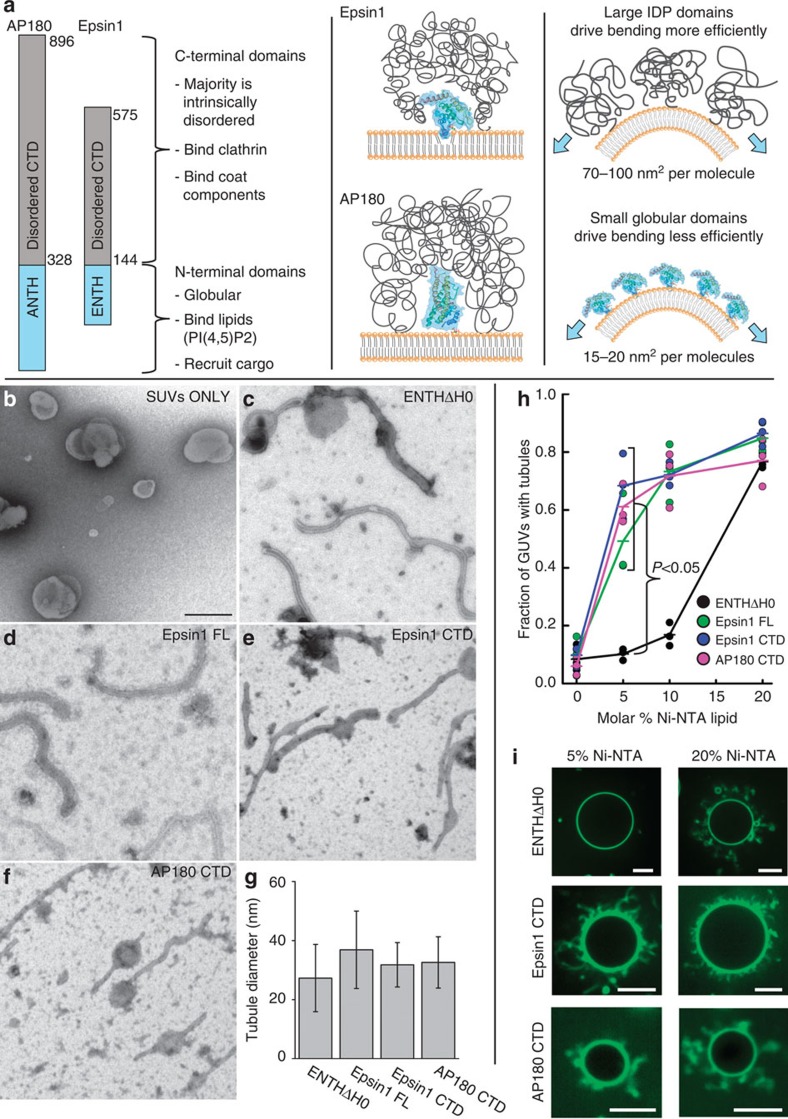
Figure 1. Disordered adaptor domains drive membrane bending efficiently.
(a) The structure of Epsin1 and AP180 consist of stably folded N-terminal domains that bind to membrane surfaces. In contrast the C-terminal domains of these adaptors are intrinsically disordered polypeptides that make multiple bonds with clathrin, other adaptor proteins and various coat components. These domains have large hydrodynamic radii in comparison with N-terminal domains, suggesting they can occupy larger areas on the membrane surface. (b–f) Transmission electron micrographs of initially spherical DOPC vesicles (SUVs) containing 20 mol% DOGS-Ni-NTA (b) mixed with the following histidine-tagged protein constructs: (c) ENTHΔH0, (d) Epsin1 FL, (e) Epsin1 CTD and (f) AP180 CTD. Scale bar is 200 nm and applies to all EM images. (g) All constructs drove membrane tubulation, with average tubule diameters in the range of 15–50 nm. Experimental data is from two electron microscope grids per protein. Error bars represent the mean±s.d.; ENTHΔH0, n=108 tubules; Epsin1 FL, n=255 tubules; Epsin1 CTD, n=121 tubules; and AP180 CTD, n=81 tubules. (h) Fraction of POPC/DOGS-Ni-NTA GUVs that form tubules following binding of histidine-tagged proteins labelled with Atto488 fluorescent dye. Proteins were added at a concentration of 5 μM. Proteins that contained disordered domains (Epsin1 FL, Epsin1 CTD and AP180 CTD) each drove the majority of GUVs to form tubules when 5 mol% DOGS-Ni-NTA was present, while the ENTHΔH0 domain required 20 mol% DOGS-Ni-NTA lipids. n=3 independent experiments, >100 GUVs per experiment. ENTHΔH0 tubulates membranes significantly less than each of the other constructs at 5 mol% DOGS-Ni-NTA (Student's t-test, unpaired two-tail, P<0.05). (i) Representative spinning disc confocal fluorescence images of GUVs containing 5 and 20 mol% DOGS-Ni-NTA after incubation with ENTHΔH0, Epsin1 CTD and AP180 CTD, respectively. Scale bar, 10 μm.