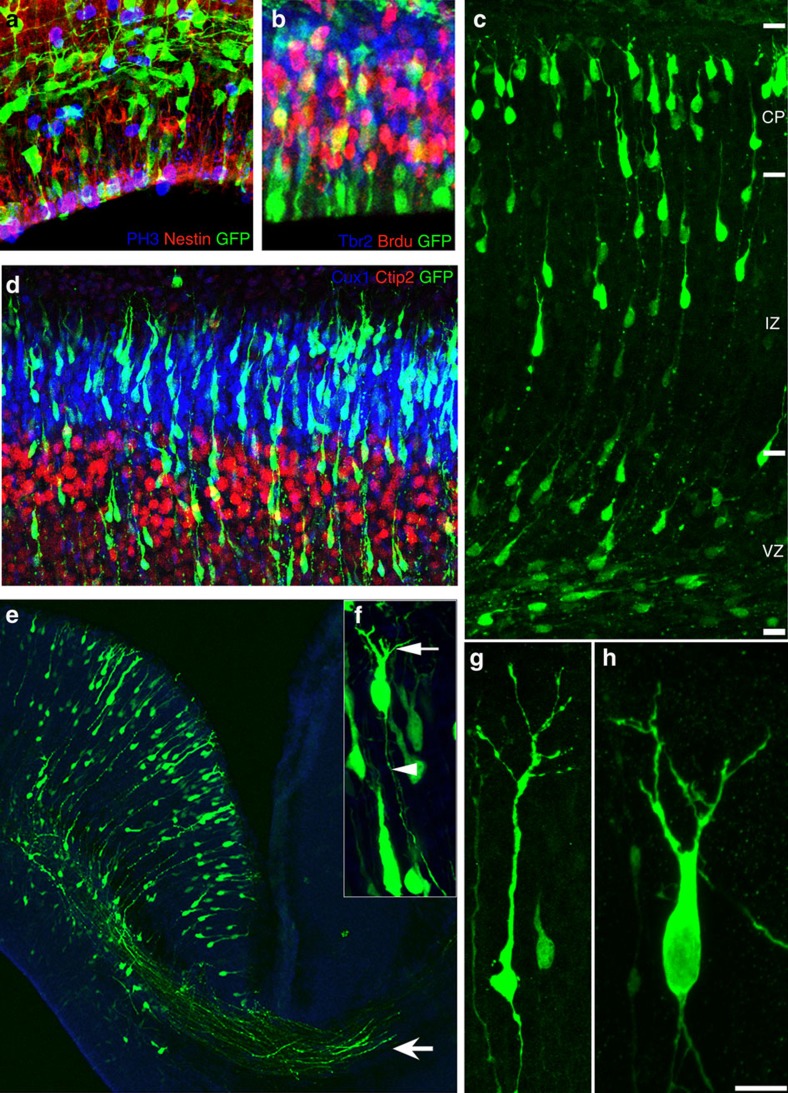
Figure 1. In utero electroporation-based assays for distinct stages of cortical development.
(a,b) Assays for cortical progenitor proliferation. shRNA-electroporated E14 embryos were allowed to survive for 2 or 4 days and shRNA-expressing (GFP+) radial progenitors were co-labelled with anti-nestin (red) and mitotic marker anti-PH3 (blue) antibodies (a). Similarly, actively proliferating intermediate progenitors were co-labelled with anti-Tbr2 (blue) and BrdU (red) in b. Changes in proliferation patterns of shRNA-expressing cells (GFP+) can be monitored in such assays. (c) Assay for neuronal migration. At 2 days after electroporation, the extent of migration of GFP+ shRNA-expressing neurons away from the ventricular zone (VZ) towards the cortical plate (CP) can be observed and measured in this assay. (d) The laminar identity of shRNA-expressing neurons in the cortical plate can be evaluated by co-labelling with different layer-specific markers. Cortical plate neurons in d were co-labelled with anti-Cux-1 (layer 2/3) and anti-Ctip2 (layer 5). (e) At E18.5, projections of the post-migratory cortical neurons to the contralateral cortex can also be visualized (arrow, e). In higher-magnification images of the cortical plate (f), the growing axon (arrowhead) and dendrites (arrow) of post-migratory neurons in the emerging cortical plate can be clearly visualized. (g,h) The developing dendritic morphology of two different post-migratory neurons. GFP+ cells in these images express control shRNA vector. The use of a ciliopathy gene shRNA library in such assays allow rapid evaluation of the role of ciliopathy-related genes in different aspects of cortical development. CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. Scale bar, 160 μm (a–c); 210 μm (d); 330 μm (e); 65 μm (f,g); 45 μm (h).