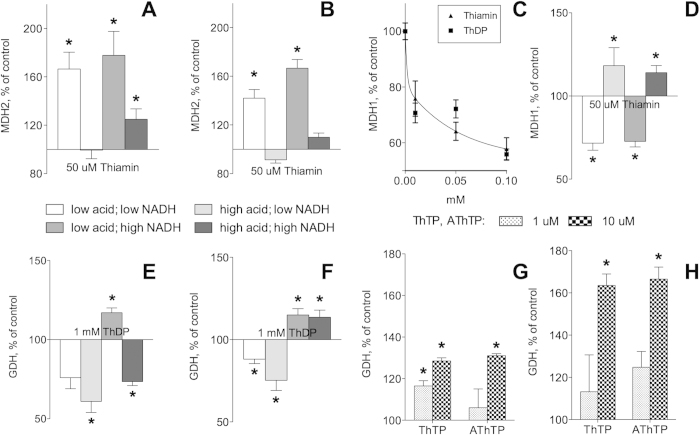
Figure 6. Regulatory effects of thiamin and derivatives on partially isolated rat brain enzymes MDH2.
(A), MDH1 (C,D), GDH (E,G) and purified porcine mitochondrial malate dehydrogenase (B) and bovine liver GDH (F,H). ThDP—thiamin diphosphate; ThTP—thiamin triphosphate; AThTP—adenylated thiamin triphosphate. (A,B,D–F)—Dependence on the substrate saturation. (C,G,H)—Dependence on concentrations of thiamin and derivatives. Shades of grey define different saturations with the dicarboxylic acid substrate (i.e. oxaloacetate or 2-oxoglutarate (2-OG), generically referred as “acid” in the figure) and NADH as indicated in the common legend to the figures (A,B,D–F). Bar patterns define varying concentrations of ThTP and AThTP as shown in the common legend to the figures (G and H). The enzymes were assayed in Ringer-bicarbonate (A–D,G,H) or 100 mM Tris-HCl buffers (E,F), pH 7.5, using the following substrate concentrations: (A,B and D)—0.01 mM oxaloacetate (low acid); 0.3 mM oxaloacetate (high acid); 0.02 mM NADH (low NADH); 0.14 mM NADH (high NADH); (C)—0.01 mM oxaloacetate, 0.02 mM NADH; (E,F)—0.1 mM 2-oxoglutarate (low acid), 2.5 mM 2-OG (high acid); 0.02 mM NADH (low NADH); 0.2 mM NADH (high NADH); G,H—0.1 mM 2-OG, 0.2 mM NADH.