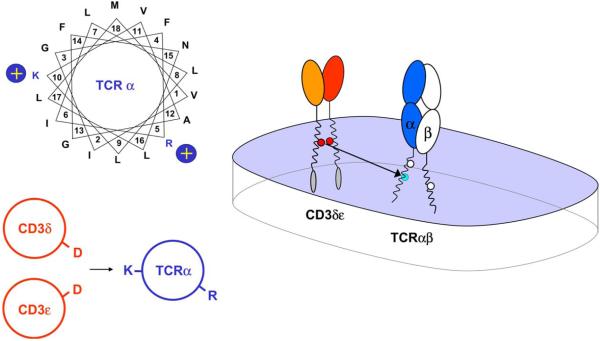
Fig. 4.
Assembly of TCRα with CD3δε through interactions between three TM helices. A helical wheel projection illustrates that the two basic TCRα TM residues are located on approximately opposite faces of the TM helix, demonstrating how they can mediate distinct assembly events. Systematic mutagenesis demonstrated that only the lysine residue (K) in the TM domain of TCRα is required for assembly with CD3δε and that the other two basic residues do not participate in this assembly step. The interaction between the TM lysine and CD3δε is highly specific since the lysine could not be substituted even by arginine. Both acidic TM residues of the CD3δε dimer are critical since mutation of either aspartic acid residue (D) greatly reduced complex formation. Assembly occurred with equal efficiency in the presence and absence of TCRβ, indicating that this assembly step results in the formation of a three-helix TM interface between TCRα and CD3δε. Such a three-helix interface can effectively shield the three ionizable residues from the lipid. This arrangement does not necessarily lead to a charge imbalance since partial or complete protonation of the acidic residues could reduce the average net charge for the aspartic acid pair.