Fig. 6.
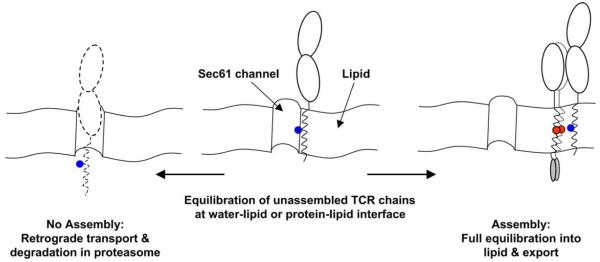
Model for coordination of assembly, export and degradation by ionizable TM residues. Work by Rapoport and colleagues (Heinrich et al., 2000) has demonstrated that TM helices with exposed basic residues are not stably integrated into the lipid bilayer. Such chains have a higher propensity to maintain an interaction with the Sec61p channel through which TM domains gain access to the hydrophobic interior of the lipid bilayer. A dynamic equilibration model can thus explain integration of TM domains into the lipid bilayer: highly hydrophobic TM domains fully equilibrate into the lipid phase, while TM domains with exposed hydrophilic residues have a greater propensity to equilibrate at the water-lipid or protein–lipid interfaces created in the vicinity of the channel. The Sec61p channel is also used for retrograde transport of proteins out of the ER, leading to their destruction in the proteasome. The dynamic equilibration model can thus explain the retention of unassembled TCR–CD3 components in the ER as well as the short half-life of individual chains in which ionizable TM residues are exposed. Assembly effectively shields these ionizable TM residues from the lipid and thus permits full equilibration of the complex into the lipid phase and export from the ER. Assembly also shields other ER retention signals, such as the retention motifs located in the C-terminal segment of the cytoplasmic domains of CD3γ and δ (Engel et al., 1992).
