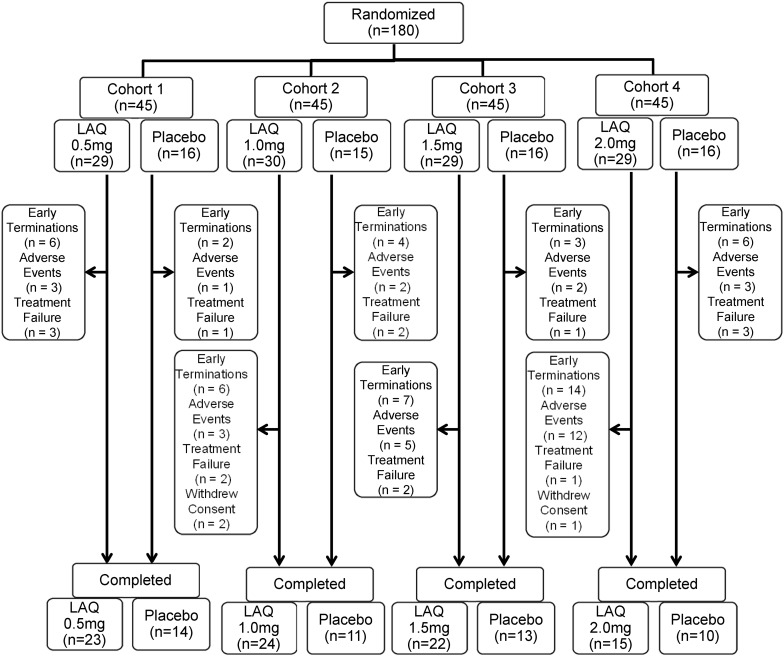
Figure 1.
Disposition of patients in the sequential cohort dose escalation study of LAQ for Crohn's disease. The number of patients completing the study and with early terminations. For early terminations, the number of patients terminated because of adverse events, treatment failure or withdrew consent is provided. LAQ, Laquinimod.