Figure 1.
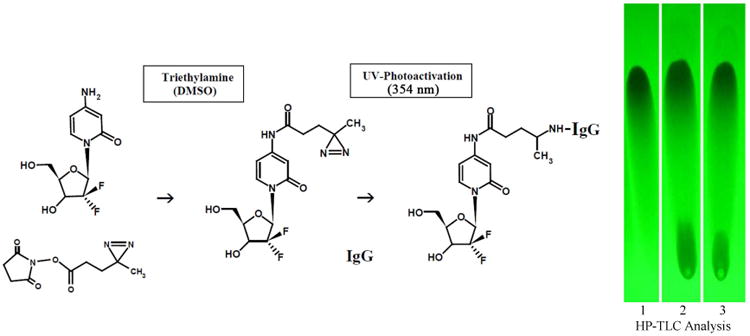
Schematic illustration of the organic chemistry reaction schemes utilized in the 2-phase synthesis regimen for gemcitabine-(C4-amide)-[anti-HER2/neu]. Legends for Reactions: (Phase-I) creation of a covalent amide bond at the C4 cytosine-like monoamine of gemcitabine and the ester group of succinimidyl 4,4-azipentanoate resulting in the creation of a covalent UV-photoactivated gemcitabine-(C4-amide) intermediate. The reaction results in the liberation of the succinimide “leaving” complex. (Phase-II) creation of a covalent bond between the UV-photoactivated gemcitabine-(C4-amide) intermediate and chemical groups within the amino acid sequence of anti-HER2/neu monoclonal immunoglobulin initiated by exposure to UV light (354 nm). Legends for HP-TLC Analysis: Reaction of the N-hydroxy-succinymide groups of disuccinimidyl glutarate with the C4 cytosine like “ring amine” of gemcitabine. (Lane-1) gemcitabine reference control; (Lane-2) gemcitabine reacted with disuccinmidyl glutarate in DMSO with Tri-ethylamide at 50 mM final concentration; and (Lane-3) gemcitabine reacted with disuccinmidyl glutarate in DMSO and ddH2O (2:1 v/v). Reaction products were developed by silica gel HP-TLC using a mobile phase of propanol/ethanol (80:20 v/v) and images visualized under UV light (254 nm).
