Figure 9.
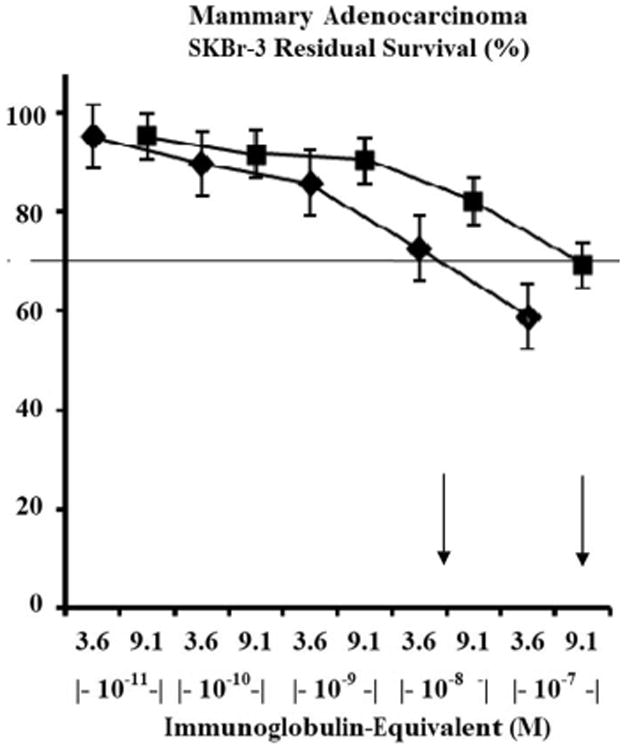
Relative cytotoxic anti-neoplastic potency of gemcitabine-(C4-amide)-[anti-HER2/neu] and gemcitabine-(C5-methylcarbamate)-[anti-HER2/neu] as a function of immunoglobulin-equivalent concentration. Legends: (4) gemcitabine-(C4-amide)-[anti-HER2/neu] with a gemcitabine molar-incorporation-index of 2.78:1 (182-hour incubation period); and (•) gemcitabine-(C5-methyl carbonate)-[anti-HER2/neu] with a gemcitabine molar-incorporation-index of 1.1:1 (182-hour incubation period). Arrows indicate the approximate concentration of gemcitabine-(C4-amide)[anti-HER2/neu] and gemcitabine-(C5-methylcarbonate)[anti-HER2/neu] necessary to achieve a 30% level of cytotoxic anti-neoplastic potentcy. Chemotherapeutic-resistant mammary adenocarcinoma (SKBr-3) monolayer populations were incubated with either covalent gemcitabine immunochemotherapeutics formulated in triplicate at gradient concentrations. Cytotoxic anti-neoplastic potency measured using a MTT cell vitality assay relative to matched negative reference controls.
