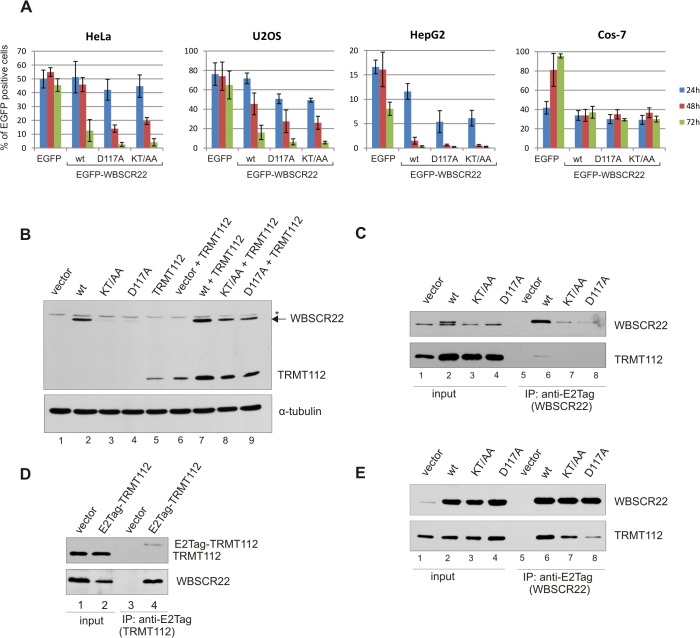
Fig 4. Expression of WBSCR22 mutants defective in TRMT112 binding.
(A) Hela, U2OS, HepG2 and COS-7 cells were transfected with plasmids expressing EGFP, EGFP-WBSCR22, EGFP-WBSCR22-KT/AA and EGFP-WBSCR22-D117A proteins. The amount of EGFP positive cells was analyzed by flow cytometry 24, 48 and 72 hours after transfection. (B) Expression of TRMT112 and WBSCR22 proteins in HeLa cells. HeLa cells were transfected with plasmids encoding for wt WBSCR22 and its mutants with and without plasmid for TRMT112. Transfected cells were harvested 24 hours after electroporation, the lysate of 105 cells was loaded on each lane and analyzed by western blot using antibody against E2Tag and α-tubulin. The non-specific signal is shown by asterisk. (C-E) Co-immunoprecipitation of WBSCR22 and TRMT112 proteins. HeLa (C) and COS-7 (E) cells were transfected with plasmids encoding for wt and mutant WBSCR22 proteins and lysed 24 hours later. (D) HeLa cells were transfected with plasmid encoding for E2Tag-TRMT112. In all cases, co-immunoprecipitation was performed using antibody against E2Tag and immunoblotting with antibodies against WBSCR22 and TRMT112. Proteins of the extract (Input; 10% of cell lysate) and pulled-down fraction (IP) were analyzed by immunoblotting.