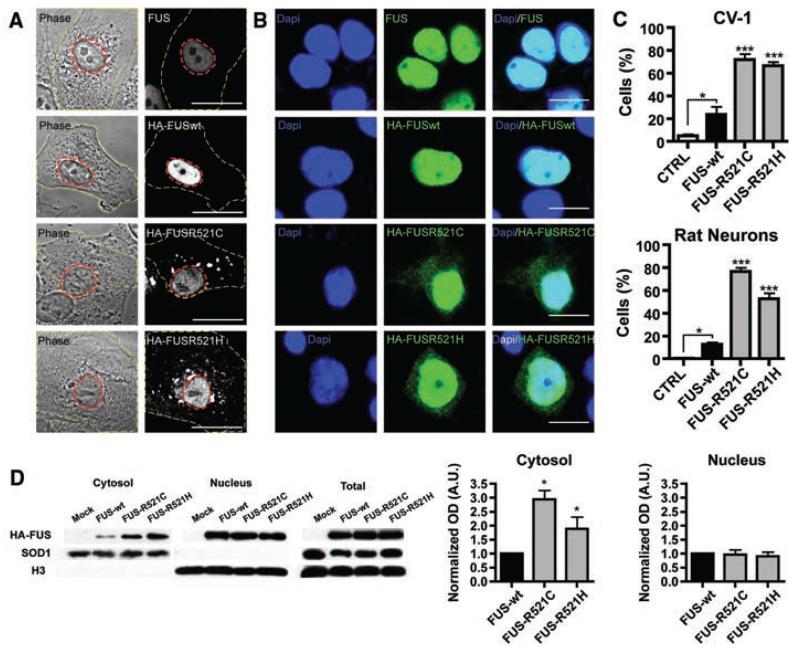
Fig. 3.
FUS mutations cause subcellular mislocalization. (A and B) The subcellular localization of endogenous FUS, transfected wild-type (HA-FUSWT), and FUS mutants (HA-FUSR521C, HA-FUSR521H) was determined in CV-1 cells (A) and rat cortical neurons (B) by immunofluorescence. The plasma membrane (A, yellow dash) and nucleus (A, red dash) of representative CV-1 cells are outlined on phase contrast (A, left panel) and corresponding FUS immunofluorescence images (A, right panel). Nuclear diamidino-2-phenylindole (DAPI) staining (B, left panel), FUS immunofluorescence (B, middle panel), and overlay (B, right panel) of representative cortical neurons are shown. Scale bars, 25 μm (A), 10 μm (B). (C) The percentage of CV-1 cells (upper panel) and cortical neurons (lower panel) showing FUS staining in the cytoplasm was significantly increased for mutant forms of FUS compared to wild-type and endogenous. Statistical significance was determined by one-way analysis of variance (P < 0.0001 for CV1 and neurons) followed by Bonferroni’s Multiple Comparison Test (*, P < 0.05; ***, P <0.001). (D) FUS mutants show a significant increase in the cytosolic fraction. N2A cells transfected with FUSWT, FUSR521C, and FUSR521H were separated into cytosolic and nuclear fractions, and the amount of transfected FUS was determined by densitometry of antibody to HA immunoblots (mean ± SEM, n = 3). The purity of fractions was determined with antibody to SOD1 (cytosol) and antibody to histone H3 (nucleus), and the transfection efficiencies were compared in total cell lysates. Statistical significance was determined by Wilcoxon Signed Rank Test (*, P < 0.05).