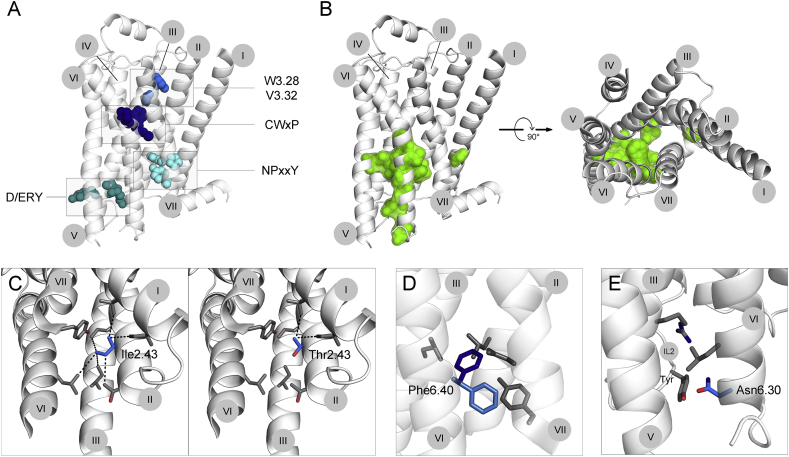
Figure 2.
Mutational alteration of GPCR basal activity. A. GPCR activation is mediated by conserved structural elements. The conserved CWxY motif and the residues 3.28 and 3.32, within the ligand binding pocket function as triggers, inducing conformational changes after ligand binding. These changes include rotameric rearrangements in the D/ERY motif in helix III and the NPxxY motif in helix VII, which stabilize the active conformation. Mutations affecting any of these essential elements are believed to alter GPCR activation. B. Mutations increasing basal activity. The residues mutated in at least two receptors are shown in green on the inactive structure of β2AR (left, side view; right, top view, as seen from the extracellular side; ECL2 helix was deleted for better visualization). C,D,E. Depending on the change in chemical properties introduced by the substituting amino acid, we propose three mechanisms, by which the mutation increases basal activity. C. Mutation of Ile2.43 to Thr2.43 decreases hydrophobicity, thereby weakening the tight helical packing. D. Introduction of Phe at position 6.40 results in physical clashes with surrounding residues and therefore probably leads to conformational changes within the helical bundle. E. Mutation of Asp6.30 to Asn changes the charge. In other receptors this Asp6.30 was reported to engage in an electrostatic interaction, which is broken by the introduction of Asn. In the β2AR this interaction rather results in repulsion with a Tyr residue in ICL2. The numbers of trans-membrane helices I-VII are indicated in gray circles.