pancreatic progenitor cells prior to endocrine induction results in beta-like cells
Schematic outlining a simplified differentiation strategy for the controlled, stepwise generation of pancreatic progenitor and subsequent endocrine cell types. GFs, growth factors.
Micrographs of differentiated clusters at day 19 under light microscopy (left picture) or fluorescent microscopy showing prominent GFP expression (right picture; GFP expression shown in white).
Quantification of the percentage of human C-peptide-positive cells at day 19–21. Values are average ± SD. n = 7 independent experiments.
Immunofluorescence stainings of differentiated clusters at day 20 for insulin (INS), PDX1, NKX6.1, NKX2.2, and glucagon (GCG). One of four experiments with similar outcome is shown.
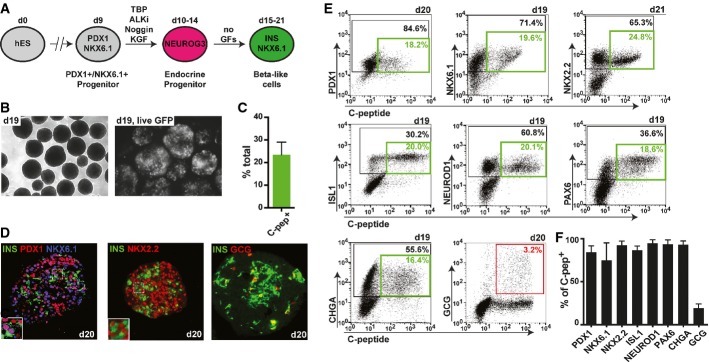
Representative flow cytometry plots depicting co-expression of pancreatic markers PDX1, NKX6.1, NKX2.2, ISL1, NEUROD1, PAX6, chromogranin A (CHGA), and GCG with human C-peptide at indicated time points. Black gates mark percentage of total cells positive for indicated marker on y-axis. Green gates mark percentage of double-positive beta-like cells. The red gate marks percentage of INS+/GCG+ bihormonal cells.
Flow cytometric quantification of C-peptide-positive beta-like cells co-expressing markers in (E). A high percentage of beta-like cells co-express all genes usually found in beta cells, but not the hormone GCG. Values are average ± SD. n = 4 for PDX1, n = 19 for NKX6.1, n = 4 for NKX2.2, n = 9 for ISL1, n = 9 for NEUROD1, n = 5 for PAX6, n = 6 for CHGA, and n = 5 for GCG. Analysis was performed at days 15–21 of differentiation.