Abstract
Exaggerated activation of lymphocytes contributes to the pathogenesis of inflammatory bowel disease (IBD). Medical therapies are linked to the BCL-2 family-mediated apoptosis. Imbalance in BCL-2 family proteins may cause failure in therapeutic responses. We investigated the role of BCL-2 inhibitor ABT-737 for lymphocyte apoptosis in mice under inflammatory conditions. B.6129P2-interleukin (IL)-10tm1Cgn/J (IL-10−/−) weighing 25–30 g with ongoing colitis were used. Fifty mg/kg/day ABT-737 was injected intraperitoneally (i.p.). Haematological analyses were performed with an ADVIA 2120 flow cytometer and mass cytometry with a CyTOF 2. Following i.p. administration, ABT-737 was detected in both spontaneous and acute colitis in peripheral blood (PBL) and colon tissue. Treatment led to lymphopenia. CD4+CD44+CD62L+ central memory and CD8+, CD44+ CD62L− central memory T cells were decreased in PBL upon ABT-737 compared to vehicle-receiving controls. Increased apoptosis upon ABT-737 was determined in blood lymphocytes, splenocytes and Peyer’s patches and was accompanied by a decrease in TNF and IL-1B. ABT-737 positively altered the colonic mucosa and ameliorated inflammation, as shown by colonoscopy, histology and colon length. A decreased BIM/BCL-2 ratio or absence of BIM in both Bim−/− and Il10−/− × Bim−/− impeded the protective effect of ABT-737. The BIM/BCL-2 ratio decreased with age and during the course of treatment. Thus, long-term treatment resulted in adapted TNF levels and macroscopic mucosal damage. ABT-737 was efficacious in diminishing lymphocytes and ameliorating colitis in a BIM-dependent manner. Regulation of inappropriate survival of lymphocytes by ABT-737 may provide a therapeutic strategy in IBD.
Keywords: ABT-737, apoptosis, BCL-2, BIM, IBD, mucosal T cell turnover
Introduction
The BCL-2 family plays a critical role in controlling immune responses by regulating the expansion and contraction of the activated lymphocyte population via apoptosis. The majority of activated T cells die at the end of a T cell response, which coincides with the exhibition of decreased levels of BCL-2 just before they begin to die in vivo 1. A decrease of the prosurvival BCL-2 contributes to apoptosis of activated T cells 2. In patients suffering from inflammatory bowel disease (IBD), the lifespan of antigen-primed and activated T cells is extended and an abnormal population of activated T cells is retained within the mucosal compartment 3–5. Enhanced expression of anti-apoptotic BCL-2 is found in lamina propria lymphocytes from inflamed Crohn’s disease (CD) tissue after CD2 pathway stimulation 3. An elevated BCL-2 level is also found in cultures of unstimulated T cells isolated from inflamed CD tissue 3. Lamina propria T cells in CD show activation of the signal transducer and activator of transcription 3 (STAT-3) signalling pathway in response to interleukin (IL)-6. STAT-3 mediates the expression of anti-apoptotic genes such as BCL-2 and BCL-XL 6. Resistance of CD T cells to apoptotic signals is correlated with an increased BCL-2 expression 3. This is supported further by reports of a higher BCL-2/BAX ratio in CD mucosa compared to controls 4 as well as a resistance to Fas-induced apoptosis in peripheral T cells acquired from CD patients 7. Apoptosis of anti-inflammatory regulatory T cells (Treg cells) is reduced in both mucosal and peripheral CD4+CD25highforkhead box protein 3 (FoxP3)+ Treg cells of IBD patients 8.
Anti-apoptotic BCL-2 interacts with the pro-apoptotic BH3-only protein family member BIM to regulate the survival of lymphocytes. The summary effect of the interaction between BCL-2 and BIM is dependent upon the cell type, but is also tissue-specific, whereby BCL-2 promotes the survival of naive T cells 9. Indeed, naive T cells from Bim+/− Bcl-2−/− mice die at an accelerated rate in vitro. BCL-2 is critical in preventing the pro-apoptotic effects of BIM in naive CD8+ T cells in vivo, but molecules other than BCL-2 may antagonize BIM in CD4+ cells. BIM controls T cell numbers in the periphery by promoting apoptosis and/or decreasing their thymic production. Bim-deficient mice have elevated numbers of normal single-positive T cells in the periphery 10. Furthermore, BIM is a primary trigger for killing B cells during their development 11. Bim deficiency prevents the death of activated T cells in vitro and in vivo, suggesting that the protective effect of BCL-2 is mediated solely by neutralization of BIM 2.
IBD affects about one in 150 people in the industrialized world. IBD is characterized by a chronic inflammation of the intestinal wall and comprises two main conditions, namely ulcerative colitis (UC) and CD. In the pathogenesis of IBD this inflammatory state is sustained by an exaggerated response of T lymphocytes to luminal antigens associated with an increased resistance to the induction of apoptosis 4.
The central elements of medical therapy consist of sulphasalazine, aminosalicylates, steroids, immunomodulators and biologicals 12,13. Medical therapies that regulate lymphocyte proliferation and contraction are linked directly to the BCL-2 family-mediated apoptosis. Sulphasalazine is a potent pro-apoptotic agent 14, as confirmed in peripheral blood lymphocytes from CD patients and in lamina propria T lymphocytes isolated from inflammatory lesions in CD patients. Induction of apoptosis by sulphasalazine is associated with a decrease in anti-apoptotic BCL-XL and BCL-2. Glucocorticoids inhibit the synthesis of IL-1 and IL-2, resulting in the suppression of the T cell-dependent immune reaction and T cell proliferation 15. Azathioprine (AZA) is an immunosuppressive agent which interferes with T cell proliferation via its metabolite 6-thioguanin-nucleotide. AZA therapy reduces natural killer cells in blood and lamina propria by increasing apoptosis 16. Increased early and late apoptosis upon AZA was determined in CD4+ T cells from peripheral blood isolated from control patients but not from CD patients 17. BCL-XL is suppressed by AZA, therefore leading to apoptosis through a mitochondrial pathway 18. Anti-tumour necrosis factor (TNF) antibodies are used in CD and UC to induce and maintain remission. Infliximab was shown to induce apoptosis and an increase in the Bax/Bcl-2 ratio of CD3/CD28 stimulated Jurkat T cells 19. Furthermore, the activation of BAX and BAK triggers apoptosis in lamina propria lymphocytes and monocytes by activating the caspase family and inducing cytochrome c release 20. Administration of all clinically effective anti-TNF antibodies resulted in a significant induction of T cell apoptosis in IBD when lamina propria CD4+ T cells expressing TNF-R2+ were co-cultured with mTNF+CD14+ intestinal macrophages 21.
Boosting the initiation of cell death in apoptosis-resistant lymphocytes could improve the success of medical therapy. ABT-737 (AbbVie; AbbVie Bioresearch (Chicago, IL, USA) is a potent inhibitor of BCL-2, BCL-XL and BCL-W 22,23 and has been shown to disrupt the interactions of BCL-2 family proteins to induce apoptosis in cancer. ABT-737 inhibits BCL-2 by directly blocking BIM-binding sites and displacing BIM, which becomes freely available to interact with other BCL-2 molecules in the cell. Additionally, BIM can bind to BAX and BAK to trigger apoptosis by initiating the formation of pores on the mitochondrial surface. ABT-737 is a BH3-only mimetic that shows a potent action against various transformed cells while exhibiting minimal toxicity towards normal cells. ABT-737 is effective in treating animal models of autoimmune diseases 24, arthritis 25 and lupus 26. Furthermore, expression of BIM, and the extent of its association with BCL-2, correlates with in vivo ABT-737 sensitivity 27.
Our own studies on apoptosis of lymphocytes in the intestinal mucosa revealed that cell death in Peyer’s patches is dependent on the pro-apoptotic protein BIM 28. Based on these findings, we investigated the role of ABT-737 in the cell death of lymphocytes in mice under inflammatory conditions.
Materials and methods
Ethical considerations
The experimental protocol was approved by the local Animal Care Committee of the University of Zurich (Cantonal Ethics Committee of Zurich, 162/2011).
ABT-737 treatment
ABT-737 was provided by AbbVie Bioresearch and was injected intraperitoneally (i.p.) at a dose of 50 mg/kg/day. The vehicle consisted of polyethylene glycol, Tween 80, dextrose solution and dimethylsulphoxide (DMSO).
Murine colitis models
Acute dextran sulphate sodium (DSS)-induced colitis.
B6.129-Bcl2l11tm1.1Ast/J (Bim−/–) mice were kindly provided by Professor Dr Andreas Villunger (Division for Developmental Immunology, Innsbruck Medical University). Bim−/− mice were back-crossed for at least 12 generations. Acute colitis was induced in female C57-BL/6J-Fue and Bim−/− mice by administration of 2% DSS for 9 days. Mice weighing 20–25 g were used for the experiments and housed in individually ventilated cages (IVC). All animals were housed for at least 3 weeks prior to testing in a specific pathogen-free (SPF) facility. Animals were killed on day 9. Acute DSS-induced colitis is an animal model for acute intestinal barrier disturbance and acute damage.
Spontaneous colitis.
B.6129P2-Il10tm1Cgn/J (Il10−/−, mice were ordered from The Jackson Laboratory, Bar Harbor, ME, USA, and were already back-crossed to the C57BL/6J genetic background for several generations) and B.6129P2-IL-10tm1Cgn/J × B6.129-Bcl2l11tm1.1Ast/J (Il10−/− × Bim−/−) develop colitis after 12–15 weeks under SPF conditions. Il10−/− weighing 25–30 g were used for the experiments. Animals were killed on day 14 of ABT-737 treatment. The IL-10−/− model of colitis reflects the exaggerated immune reaction against the endogenous gut flora as seen in CD patients. Il10−/− have a low penetrance of disease when raised under SPF conditions, and therapeutic studies in this model are difficult to perform. Therefore we defined a clearly visible rectal prolapse as an external visible indicator of ongoing colitis in our Il10−/− colony, as also stated in the Results section, whenever Il10−/− suffering from ongoing colitis were used.
Assessment of colonoscopy and histological score in mice
Animals were anesthaetized with isoflurane and examined with the Tele Pack Pal 20043020 (Karl Storz Endoskope, Tuttlingen, Germany) and scored according to the murine endoscopic index of colitis severity (MEICS), as described previously 29. For assessment of the histological scores, 1 cm of the distal third of the colon was removed and scored as described 30,31.
Cell isolation
Murine peripheral blood lymphocytes were used for mass cytometric analyses. Whole blood was collected in ethylenediamine tetraacetic acid (EDTA)-treated collection tubes (BD vacutainer, ref. 367525; BD Biosciences, San Jose, CA, USA). ACK (1·5 M NH4Cl, 100 mM KHCO3, 10 mM Triplex111, pH 7·2) lysing buffer was used for lysing red blood cells.
Splenocytes were isolated using the gentle magnetic affinity cell sorting (gentleMACS) octo dissociator (Miltenyi Biotec, San Diego, CA, USA; no. 130-095-937) and gentleMACS C tubes (Miltenyi Biotec; no. 130-093-237). One mouse spleen was transferred into a gentleMACS C tube containing 10 ml ACK. A single-cell suspension was obtained by using the gentleMACS octo dissociator program m_spleen 03 three times. Splenocytes were passed over a 70-μm mesh filter and centrifuged. Cells were resuspended in 1 ml ACK, centrifuged, resuspended in phosphate-buffered saline (PBS) and passed again over a 70-μm mesh filter to obtain 200–300 × 106 splenocytes/spleen.
Lamina propria mononuclear cells (LPMNC) from small bowel were isolated with the help of the lamina propria dissociation kit (Miltenyi Biotec; no. 130-097-410), according to the manufacturer’s protocol.
Mass cytometry, staining, data acquisition and analysis
Single-cell suspensions obtained from peripheral blood, spleen and lamina propria were stained according to the manufacturer’s recommendations (MaxPar Cell Surface Staining Protocol and MaxPar Cytoplasmic/Secreted Antigen Staining Protocol; DVS Sciences, Toronto, Canda). In short, for viability staining cells were washed with PBS and resuspended in PBS to a maximum concentration of 1 × 107/ml. Cell-ID cisplatin reagent (Fluidigm, San Francisco, CA, USA; cat. no. 201064) was added to a final concentration of 5 µM. Following 5 min incubation at room temperature, samples were washed with cell staining buffer (Fluidigm; cat. no. 201068) by adding 5× the volume of the initial cell suspension. Each sample was resuspended in a volume of 50 µl cell staining buffer and 50 µl of the surface antibody cocktail was added (MaxPar Mouse Spleen/Lymph Node Phenotyping Panel Kit, cat. no. 201306). Following 30 min incubation at room temperature, samples were washed twice with 2 ml of cell staining buffer each and fixed by adding 1 ml of 1× MaxPar Fix I Buffer (Fluidigm; cat. no. 201065) to each tube, gently vortexed and incubated at room temperature for an additional 15 min. Cells were washed twice with 2 ml MaxPar Perm-S buffer each (Fluidigm; cat. no. 201066) and resuspended in 50 µl of the same buffer. The intracellular antibody cocktail (MaxPar Mouse Intracellular Cytokine I Panel Kit, cat. no. 201310) was added to each sample in 50 μl volume and incubated for 30 min at room temperature. Following two washing steps with 2 ml of cell staining buffer each, cells were labelled with 1 ml of 100 nM iridium intercalation solution (Fluidigm; cat. no. 201192B) in MaxPar Fix and Perm buffer (Fluidigm; cat. no. 201067) at 4 °C overnight. Cells were washed twice with 2 ml cell staining buffer each and once with water (Ultrapure Mili-Q water, 18·2 MΩ*cm at 25°C; Merck Milipore, Darmstadt, Germany). Cells were left pelleted until ready for acquisition, at which point their concentration was adjusted to 1–1·5 × 106 cells/ml in the Ultrapure Mili-Q water.
Cells were acquired on the CyTOF 2 instrument (Fluidigm) with an acquisition flow rate of 0·045 ml/min and following data processing settings (default thresholding scheme, lower convolution threshold of 200 IU, minimum event duration of 12 pushes, maximum event duration of 100 pushes, noise reduction active). All samples were spiked with EQ four-element calibration beads during acquisition (Fluidigm; cat. no. 201078) and resulting FCS files were normalized according to the built-in normalization algorithm (CyTOF software version 6·0·626) to account for intra- and intersample intensity measurement variability. The resulting data were analysed with FlowJo (Flowjo LLC; TreeStar, Inc., Ashland, OR, USA) and Cytobank (Cytobank Inc., Mountain View, CA, USA) software.
Blood analyses
Haematological analyses were performed by the Division of Hematology at the University Hospital Zurich with an ADVIA 2120 flow cytometer (Siemens, Eschborn, Germany).
RNA extraction and quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Basel, Switzerland). mRNA was reverse-transcribed into cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Waltham, MA, USA). Bim (no. Mm00437796_m1; Applied Biosystems), Bcl-2 (no. Mm00477631_m1*), TNF (no. Mm99999068_m1) and IL1b (no. Mm01336189_m1) gene expression was determined with a TaqMan® gene expression assay. Actin gene expression was measured as endogenous control (no. 4352341E; Applied Biosystems) and used for the calculation of relative mRNA expression using the ΔΔCt method. All samples were analysed as triplicates.
Analysis of apoptosis and flow cytometry
Apoptosis was quantified by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) technology with the In Situ Cell Death Detection Kit (no. 11684795910; Roche, Mannheim, Germany), as described by the manufacturer.
Numbers of living and apoptotic cells were determined by flow cytometry of annexin V- and propidium iodide (PI)-stained cells. Cells were resuspended in annexin V binding buffer, as indicated by the manufacturer, and stained with allophycocyanin (APC)-conjugated annexin V (Enzo Life Science, Basel, Switzerland; diluted 1 : 40) and PI (Sigma-Aldrich, St Louis, MO, USA; final concentration 25 µg/ml). Annexin V−/PI− cells were considered alive; apoptotic cells displayed staining for annexin V but not for PI.
Fluorescence was measured by flow cytometry using a BD fluorescence activated cell sorter (FACS)Canto II flow cytometer equipped with two lasers (excitation wave lengths: 488 and 633 nm). CD4 was stained with anti-mouse antibody from ImmunoTools (no. 22150043sp, 1 : 1000). CD8 was stained with anti-mouse antibody from ImmunoTools (Friesoythe, Germany; no. 22150083sp, 1 : 1000). Both primary antibodies were directly conjugated with fluorescein isothiocyanate (FITC).
Statistical analyses
Real-time PCR data were calculated from triplicates. Statistical analyses were performed using PASW statistics version 18·0 (SPSS Inc., Chicago, IL, USA). Kruskal–Wallis non-parametric analysis of variance and Bonferroni-corrected Mann–Whitney rank-sum tests were applied for animal experiments. Box-plots express median with relative quartiles, minimum and maximum. One-way analysis of variance (anova) and Tukey’s post-hoc test were used for cell culture experiments. Bars represent mean values with whiskers displaying standard deviation. Differences were considered significant at P < 0·05 (*), highly significant at P < 0·01 (**) and very highly significant at P < 0·001 (***).
Results
ABT-737 is detected in blood and intestine following i.p. administration
ABT-737 was applied in the Il10−/− model of spontaneous colitis. A calculated dose of 50 mg/kg/day was injected i.p. for 14 days. Control mice received a corresponding dose of vehicle solution during the same time-period. No treatment-related death or evidence of infection was observed in any of the animals. Water consumption was not reduced in mice treated with ABT-737 compared to controls. Peripheral blood, faeces, intestinal epithelial cells (IEC) and whole colon tissue of mice were harvested (n > 4 each). Using mass spectrometric (MS) quantification, ABT-737 could be detected in the peripheral blood. Following 14 days of treatment, ABT-737 was localized in peripheral blood (0·47 ± 0·26 μg/μl), faeces (9·44 ± 4·12 μg/g), IECs (6·07 ± 6·20 μg/g) and whole colon tissue (3·67 ± 3·91 μg/g). This confirms that following i.p. administration ABT-737 indeed reaches the peripheral blood. MS quantification proved the presence of ABT-737 in whole colon tissue, its uptake into IEC and its accumulation in the faeces.
Additionally, ABT-737 was applied in the murine model of acute DSS-induced colitis; 50 mg/kg/day was injected i.p. for 9 days. By MS quantification, ABT-737 could be detected in the peripheral blood (0·74 ± 0·37 μg/μl), faeces (29·96 ± 21·17 μg/g), IECs (1·61 ± 2·90 μg/g) and whole colon tissue (7·17 ± 0·93 μg/g).
ABT-737 induces a significant lymphopenia in mouse models of colitis
Flow cytometry was performed to quantify viability of lymphocytes upon absorbed ABT-737 in Il10−/− mice. The relative number of live peripheral blood lymphocytes (PBL) was reduced significantly in Il10−/− upon ABT-737 compared to controls (45·4 ± 17·4%, n > 8 versus 82·4 ± 4·8%, n > 8, respectively, P < 0·05, Fig. 1a). Haematological analyses were performed in the Il10−/− model of spontaneous colitis to determine whether the significant increase in number of apoptotic cells upon ABT-737 was associated with a shift in cell populations. The lymphocyte fraction was reduced significantly in Il10−/− mice after 14 daily injections of ABT-737 when compared to vehicle-receiving controls (45·9 ± 16·1%, n > 8 versus 67·2 ± 14·0%, n > 8, respectively, P < 0·05, Fig. 1b). The absolute number of lymphocytes was reduced significantly in Il10−/− upon ABT-737 compared to controls (0·53 ± 0·37 × 103 cells/μl, n > 8 versus 1·83 ± 0·83 × 103 cells/μl, n > 8, respectively, P < 0·05). In accordance, the combined granulocyte and monocyte-fraction was increased significantly in ABT-737-treated Il10−/− mice (49·3 ± 14·8%, n > 8 versus 24·6 ± 11·1%, n > 8, respectively, P < 0·05, Fig. 1c). The absolute number of the combined granulocyte and monocyte-fraction was reduced in Il10−/− upon ABT-737 compared to controls (0·54 ± 0·18 × 103 cells/μl, n > 8 versus 0·88 ± 0·48 × 103 cells/μl, n > 8, respectively), although not significantly different. In contrast, erythrocyte counts were not affected (9·13 ± 0·83 × 1012 cells/μl, n > 8 versus 8·96 ± 0· 87 × 1012 cells/μl, n > 8). As inhibition of BCL-2 by ABT-737 is mediated by displacing the pro-apoptotic factor BIM, we also applied ABT-737 in Il10−/− × Bim−/−. Both the fraction of lymphocytes (78·8 ± 10·1%, n > 5 versus 81·2 ± 7·5%, n > 4, respectively, Fig· 1b) and of granulocytes and monocytes (10·9 ± 7·8%, n > 5 versus 12·8 ± 4·3%, n > 4, respectively, Fig. 1c) were unaltered upon ABT-737. The absolute number of lymphocytes remained unchanged in Il10−/− × Bim−/− upon ABT-737 compared to controls (6·16 ± 3·61 × 103 cells/μl, n > 5 versus 5·47 ± 3·81 × 103 cells/μl, n > 5, respectively), as well as the absolute number of the combined granulocyte and monocyte-fraction (0·90 ± 1·22 × 103 cells/μl, n > 5 versus 1·08 ± 0·71 × 103 cells/μl, n > 5, respectively). PBL from colitic Il10−/− mice were analysed by mass cytometry. T cell receptor (TCR)-β+, CD3+ and CD4+ were analysed for CD44 and CD62L expression (Fig. 1d). The proportion of CD44+CD62L+ central memory T cells was decreased upon ABT-737 (10·4 ± 7·4%, n > 3) compared to vehicle-receiving controls (23·8 ± 8·1, n > 3). The proportion of TCR-β+, CD3+, CD8+ and CD44+CD62L− central memory T cells was decreased upon ABT-737 (17·9 ± 11·5%, n > 3) compared to vehicle-receiving controls (34·2 ± 24·0, n > 3).
Figure 1.
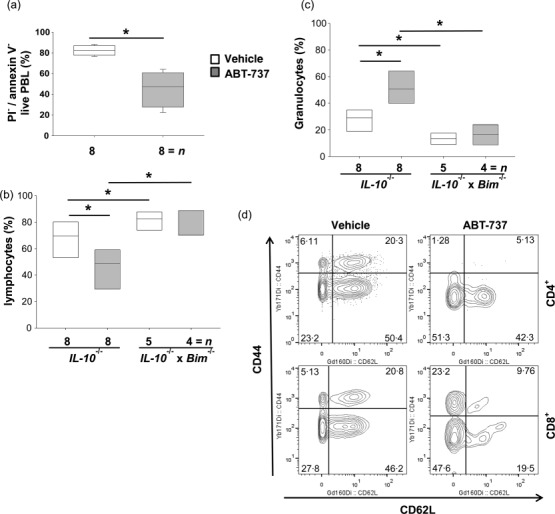
Cell death upon ABT-737 is selective for peripheral blood (PBL). (a–d) Il10−/− and Il10−/− × Bim−/− mice in the model of spontaneous colitis. (a) Flow cytometry revealed an increased number of propidium iodide (PI)+ cells in PBL upon ABT-737 treatment. Mann–Whitney rank-sum test. Treatment with ABT-737 (b) significantly decreased the number of lymphocytes and (c) significantly increased the number of granulocytes compared to vehicle. Statistical analysis was performed using analysis of variance (anova) and all pairwise multiple comparison procedures (Bonferroni t-test), P < 0·05 (*). (d) Mass cytometry revealed a decreased proportion of CD44+ CD62L+ central memory T cells upon ABT-737 compared to vehicle.
Additionally, haematological analyses for C57-BL/6J-Fue mice with a DSS-induced acute colitis confirmed a significantly reduced absolute lymphocyte count upon ABT-737 compared to controls (Supporting information, Fig. S1a). ABT-737 levels in peripheral blood determined by MS were correlated directly to the removal of lymphocytes, as well as an increased relative number of granulocytes and monocytes (Supporting information, Fig. S1c). The absolute number of the combined granulocyte and monocyte-fraction was reduced in mice suffering from DSS-induced colitis upon ABT-737 compared to controls (1·04 ± 0·68 × 103 cells/μl, n > 12 versus 1·42 ± 1·04 × 103 cells/μl, n > 12, respectively), although not significantly different. In flow cytometry, CD8+ appeared to be diminished upon ABT-737 treatment, although the differences were not significant (7·8 ± 3·2%, n > 10 versus 10·7 ± 6·2%, n > 12, respectively).
The decrease in lymphocyte counts is accompanied by a reduction of proinflammatory cytokines
We determined whether the significant decrease of lymphocyte counts in Il10−/− mice was associated with a decrease in TNF and IL1b gene expression upon ABT-737 treatment. The cytokine profile of Il10−/− mice was assessed in peripheral blood drawn from the tail vein on days 7 and 14. TNF gene expression was decreased significantly on day 14 in ABT-737-treated mice compared to vehicle-treated mice (82 ± 28, n > 8 versus 241 ± 15, n > 8, respectively, Fig. 2a). IL1b gene expression was also decreased significantly on day 14 in ABT-737-treated mice (49 ± 27, n > 8 versus 276 ± 135, n > 8, respectively, Fig. 2b). With regard to Il6 and Ifng mRNA expression, no significant changes were recorded.
Figure 2.
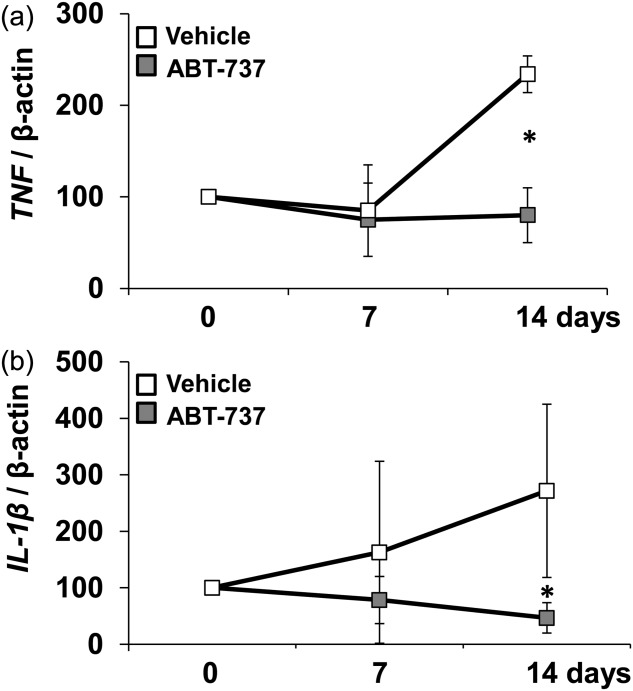
During spontaneous colitis in interleukin (IL)-10−/− proinflammatory cytokines were decreased significantly upon ABT-737 treatment compared to vehicle-receiving controls. mRNA expression levels determined by quantitative polymerase chain reaction (qPCR) from (a) tumour necrosis factor (TNF) and (b) IL-1b. Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*).
BIM is required for the protective effect of ABT-737
Splenocytes from colitis Il10−/− mice were analysed by flow cytometry after 14 daily injections of ABT-737. ABT-737 treatment was followed by a significant decrease in CD8+ splenocytes in Il10−/− mice compared to vehicle-receiving Il10−/− mice (15·9 ± 3·3%, n > 9 versus 9·2 ± 2·5%, n > 9, respectively, P < 0·05, Fig. 3a,b). To elucidate ABT-737-dependent changes in apoptosis in the absence of BIM, Bim−/− × Il10−/− mice were used. In contrast to the results obtained from Il10−/− mice, there was no detectable difference in CD8+ splenocytes from Bim−/− × Il10−/− mice upon ABT-737 (Fig. 3a). This significant decrease in CD3+ CD8+ splenocytes in Il10−/− mice upon ABT-737 compared to vehicle-receiving Il10−/− mice (17·5 ± 1·1%, n > 3 versus 11·5 ± 1·9%, n > 3, respectively, P < 0·05; statistical analysis was performed using the Holm–Sidak method, Fig. 3c) was also confirmed by mass cytometry.
Figure 3.
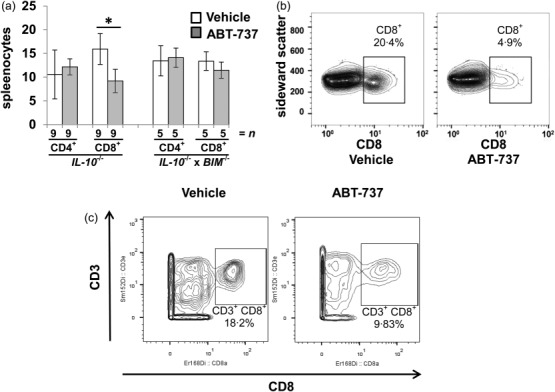
Increased apoptosis in the spleen upon ABT-737 treatment. (a–c) Interleukin (IL)-10−/− mice suffering from spontaneous colitis. (a) Decreased number of CD8+ splenocytes in Il10−/− upon ABT-737 compared to vehicle determined by flow cytometry. Number of CD4+ and CD8+ splenocytes in Bim−/− × Il10−/− remained unchanged upon ABT-737. Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*). (b) Decreased number of CD8+ splenocytes in Il10−/− upon ABT-737 compared to vehicle determined by flow cytometry. (c) Mass cytometric analysis of T cell receptor (TCR)β+ splenocytes revealed a significantly decreased proportion of CD3+CD8+ T cells upon ABT-737 compared to vehicle. Statistical analysis was performed using the Holm–Sidak method, P < 0·05 (*).
Apoptosis of splenocytes is increased significantly upon ABT-737 treatment
Increased apoptosis in peripheral blood was associated with a reduction of lymphocyte counts in ABT-737-treated mice. The level of cleavage of genomic DNA in spleen in the DSS-induced acute colitis model was analysed. TUNEL assays revealed an increased apoptosis in mice treated for 9 days with ABT-737 (Supporting information, Fig. S2a,b). Apoptosis-positive cells were detectable in the red pulp and to a lesser extent in the white pulp.
Furthermore, ABT-737 treatment was followed by a significant reduction in spleen weight in C57-BL/6J-Fue mice suffering from DSS-induced acute colitis (0·10 ± 0·03 g for vehicle-receiving mice compared to 0·07 ± 0·02 g for ABT-737-receiving mice; Supporting information, Fig. S2c,d). This difference in spleen weight was not seen in colitic Bim−/− mice upon ABT-737 treatment. Irrespective of the treatment, spleen weight in Bim−/− was increased compared to C57-BL/6J-Fue mice, as described previously (Supporting information, Fig. S2c).
ABT-737 increases apoptosis significantly in Peyer’s patches
The increased number of TUNEL+ cells in the peripheral blood and spleen indicated that the number of lymphocytes was reduced in ABT-737-treated mice. Additionally, significantly increased cleavage of genomic DNA was found in Peyer’s patches in the Il10−/− model in both groups treated for 5 (Supporting information, Fig. S3a) and 14 days (Fig. 4a,b) with ABT-737. Next we isolated LPMNC from small bowel from Il10−/− treated for 14 days. Viable cells were identified by cisplatin staining followed by mass cytometry. A certain number of dead cells is certainly due to the isolation procedure; however, a significantly increased number of dead cells was found in LPMNC isolated from small bowel in the Il10−/− model with ABT-737 compared to vehicle-treated mice (72·9 ± 3·1%, n > 3 versus 46·8 ± 12·2%, n > 3, respectively, P < 0·05, Holm–Sidak method, Fig. 4c). A decrease in viable CD3+CD4+ LPMNC in Il10−/− mice upon ABT-737 compared to vehicle-receiving Il10−/− mice (4·9 ± 3·8%, n > 3 versus 13·3 ± 6·4%, n > 3, respectively, P < 0·05, Fig. 4d) was confirmed by mass cytometry. Additionally, a decrease in viable CD3+CD8+ LPMNC upon ABT-737 (3·6 ± 2·7%, n > 3 versus 11·2 ± 6·4%, n > 3, respectively, P < 0·05, Fig. 4d) was found.
Figure 4.
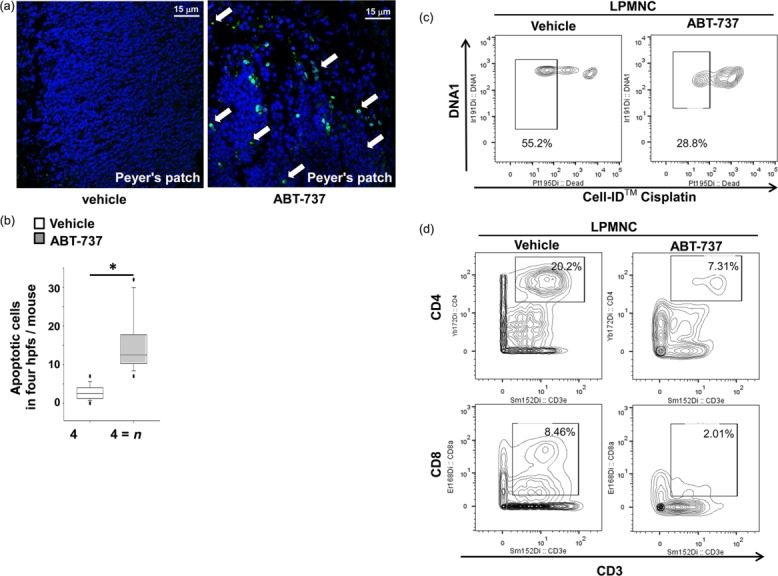
Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737. (a) Il10−/− mice were killed at day 14; original magnification × 630. Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*). (b) The number of cells was calculated from four high-power fields for each mouse. (c) Mass cytometric analysis of of lamina propria mononuclear cells (LPMNC) revealed a significant decrease in dead cells upon ABT-737 compared to vehicle. Statistical analysis was performed using the Holm–Sidak method, P < 0·05 (*). (d) Mass cytometric analysis LPMNC revealed a decrease in both CD3+CD4+ and CD3+CD8+ T cells upon ABT-737 compared to vehicle.
Although mice suffering from acute DSS-induced colitis do not develop ileitis, TUNEL staining also revealed increased apoptosis in Peyer’s patches from mice treated for 9 days with ABT-737 upon water (Supporting information, Fig. S4) or DSS (Supporting information, Fig. S5). TUNEL also revealed an increased apoptosis in the lamina propria of mice treated for 9 days with ABT-737 (Supporting information, Figs S4 and S5). TUNEL+ cells appeared more frequently as clusters of more than four cells (Supporting information, Fig. S6).
ABT-737 ameliorates spontaneous and acute colitis
To determine the influence of ABT-737 dependent on mucosal inflammation and apoptosis both the Il10−/− model and the DSS-induced model were used. Macroscopic mucosal damage was assessed by mini-endoscopy. Il10−/− mice with a clearly visible rectal prolapse received 50 mg/kg/day (n > 18) or vehicle (n > 14) for 14 days. Mice were killed on day 14. Il10−/− mice receiving vehicle displayed an opaque mucosa and altered vascular pattern on day 14 (MEICS 6·9 ± 2·3, n > 14, Fig. 5a). The thickening of the colon became more prominent, thin faeces were discovered and the diseased regions presented frequently with mucosal bleeding. During treatment with ABT-737, inflammation was ameliorated. Compared to vehicle-receiving mice, Il10−/− mice treated with ABT-737 had a transparent mucosa with a regular vascular pattern. Solid faeces were visible (MEICS 3·6 ± 1·7, n > 18, Fig. 5b). No differences in MEICS were determined in Il10−/− × Bim−/− upon ABT-737 (Fig. 5a,b).
Figure 5.
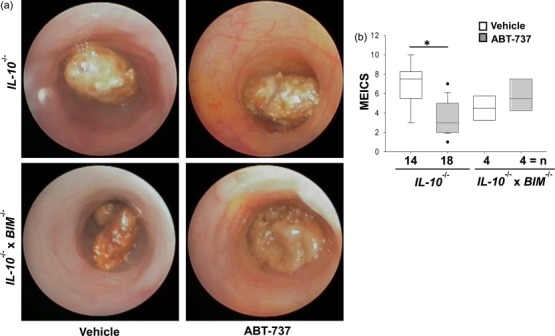
Mice suffering from colitis develop an ameliorated intestinal inflammation upon ABT-737 treatment compared to vehicle-receiving mice. (a,b) Il10−/− mice suffering from spontaneous colitis. (a) Representative images demonstrate colonoscopy pictures derived from ABT-737- and vehicle-treated mice. (b) Statistical analysis of colonoscopy score [murine endoscopic index of colitis severity (MEICS)]. Normality test was passed and statistical analysis was performed using one way analysis of variance (anova) (Bonferroni t-test). Colonoscopy was performed on the day prior to killing the animals. Box-plots express median, 95/5% percentiles and outliers, P < 0·05 (*).
Additionally, upon acute colitis, mice receiving vehicle displayed an opaque mucosa and altered vascular pattern on day 9 (MEICS 4·3 ± 1·8, n > 14; Supporting information, Fig. S7). Colon became thickened, extensive amounts of faeces were discovered and the diseased regions presented frequently with mucosal bleeding. During treatment with ABT-737 signs of an ameliorated inflammation were found. Mice suffering from DSS-induced acute colitis but treated with ABT-737 had a transparent mucosa with a regular vascular pattern. Solid faeces were visible (MEICS 1·4 ± 0·9, n > 13; Supporting information, Fig. S7). In contrast, no difference in MEICS was determined in Bim−/− mice treated with ABT-737 (Supporting information, Fig. S7). In summary, ABT-737 positively altered the colonic mucosa at a macroscopic level in both the Il10−/− model of spontaneous colitis and DSS-induced acute colitis.
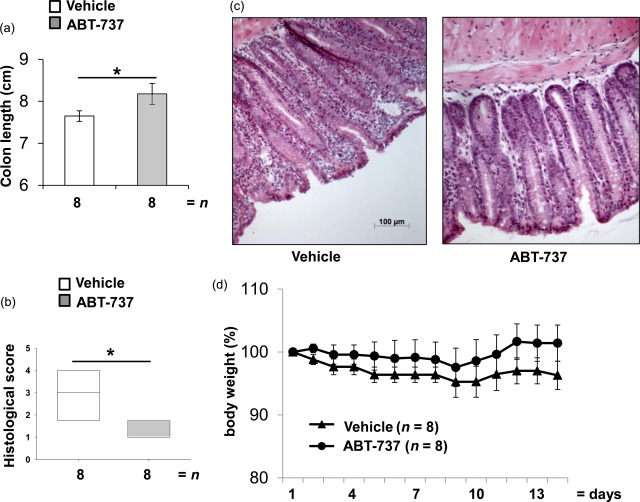
Colon length was determined in both spontaneous colitis and DSS-induced acute colitis models. In Il10−/− mice, ABT-737 treatment prevented the shortening in colon length significantly (8·18 ± 0·27 cm versus 7·65 ± 0·38 cm, respectively, Fig. 6a). The histological score of Il10−/− mice treated with ABT-737 was significantly lower, as in untreated mice (Fig. 6b). ABT-737-treated Il10−/− mice had less lymphocyte influx in the thickened lamina propria (Fig. 6c). Compared to vehicle-receiving mice, Il10−/− mice treated with ABT-737 showed increased body weight (Fig. 6d), although not significantly different.
Figure 6.
ABT-737 treatment was followed by a significant increase of the colon length. (a–d) Il10−/− mice suffering from spontaneous colitis. (a) In Il10−/− mice ABT-737 treatment was followed by a significant increase in colon length. Statistical analysis was performed using the Mann–Whitney rank-sum test. (b) Histological score was decreased significantly upon ABT-737 treatment. Statistical analysis was performed using the Mann–Whitney rank-sum test. Box-plots express median. (c) Haematoxylin and eosin (H&E) staining of distal colon. Images representative for n > eight each, P < 0·05 (*); P < 0·001 (***). (d) Body weight curve.
In C57-BL/6J-Fue mice, induction of DSS-induced acute colitis was followed by a significant reduction in colon length (Supporting information, Fig. S8a). ABT-737 treatment prevented the shortening in colon length significantly in DSS-induced colitis (7·44 ± 0·51 cm, n > 18 versus 6·35 ± 0·57 cm, n > 18, P < 0·001, respectively; Supporting information, Fig. S8a). The number of lymphocytes in colonic mucosa was determined and immunohistochemistry applying anti-CD3 antibody was performed. CD3+ cells in the acquired images were marked appropriately and quantified by an investigator blinded to the experiment setup. Immunohistochemistry revealed a decreased number of CD3+ in the colonic lamina propria of mice treated for 9 days with ABT- [49·5 ± 39·5 cells/high-power field (hpf), n > 16 hpfs and 70·1 ± 30·4 cells/hpf, n > 14 hpfs, respectively; Supporting information, Fig. S8b].
CD4+CD62L+ are susceptible for priming with ABT-737 but Bcl-2 increases during ongoing inflammation
We assessed ABT-737-dependent priming for apoptosis in lymphocyte subpopulations. Il10−/− with a clearly visible rectal prolapse received either a dose of 50 mg/kg/day ABT-737 (n > 5) or vehicle (n > 5) for 14 days as in-vivo pretreatment. CD4+CD62L+ were isolated from the splenocytes and incubated for 12 h with ABT-737 or vehicle. Following ABT-737 in-vivo pretreatment CD4+CD62L+ cells became significantly more susceptible to apoptosis initiated by ABT-737 compared to vehicle controls (Fig. 7a). According contour plots are shown in Supporting information, Fig. S9.
Figure 7.
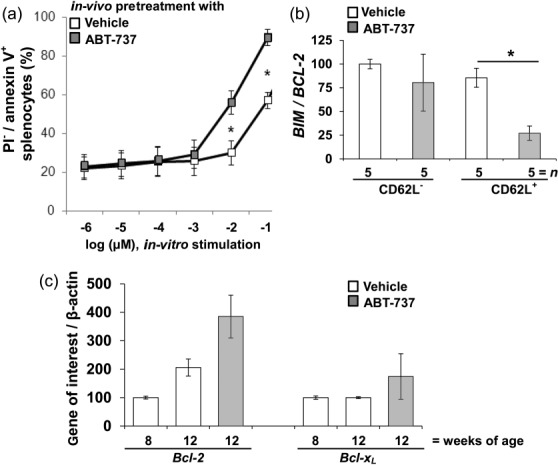
Significantly increased apoptosis in CD4+CD62L+ from Il10−/− mice upon ABT-737 pretreatment compared to vehicle. (a) CD4+CD62L+ cells were isolated from Il10−/− mice treated for 14 days with ABT-737 or vehicle in vivo. CD4+CD62L+ cells were stimulated in vitro with ABT-737 or vehicle in a dose-dependent manner. Following ABT-737 pretreatment, cells became significantly more susceptible to the initiation of apoptosis as determined by flow cytometry. (b) The BIM/BCL-2 ratio was decreased significantly in CD4+CD62L+ from ABT-737-treated mice compared to vehicle-treated mice. (c) BCL-2 and BCL-xL quantitative polymerase chain reaction (qPCR) of splenocytes isolated from Il10−/−. Gene expression was increased in a time-dependent manner and upon ABT treatment compared to vehicle-receiving controls. Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*).
Furthermore, BIM and BCL-2 gene expression in CD4+CD62L+ and CD4+CD62L− cells after pretreatment with either ABT-737 or vehicle was determined. The BIM/BCL-2 ratio was decreased significantly on day 14 in CD4+CD62L+ from ABT-737-treated mice compared to vehicle-treated mice (26 ± 8, n > 5 versus 87 ± 13, n > 5, respectively, Fig. 7b). In-vivo pretreatment with ABT-737 lowered the BIM/BCL-2 ratio in CD4+CD62L+. As the BIM/BCL-2 ratio was decreased significantly on day 14 in CD4+CD62L+ from ABT-737-treated mice, BCL-2 and BCL-XL gene expression in CD4+CD62L+ cells in relation to age and treatment was determined. CD4+CD62L+ from IL-10−/− 8 weeks of age (n > 5) were compared to 12-week-old mice with (n > 5) and without ABT-737 treatment (n > 5). Eight-week-old mice without visible rectal prolapse were compared with 12-week-old mice with either a visible or a developing rectal prolapse. BCL-2 gene expression was increased significantly in older mice compared to younger mice. BCL-2 was also increased significantly upon ABT-737 compared to vehicle control (Fig. 7c).
Apoptosis stimulating effect is decreased upon ABT-737 long-term treatment
Long-term treatment with ABT-737 in Il10−/− mice was performed. Il10−/− at 8 weeks of age, with no signs of rectal prolapse, received either 50 mg/kg ABT-737 every 3 days (n > 10) or vehicle (n > 10) during a period of 56 days. Treatment was applied i.p. Long-term treatment with ABT-737 induced depigmentation of the abdominal skin, whereas the head, chest and back were not affected (Supporting information, Fig. S10).
Il10−/− were killed on day 56. Seven mice upon ABT-737 and eight vehicle-treated mice developed a clearly visible rectal prolapse during the experiment. Macroscopic mucosal damage was assessed by mini-endoscopy and colonoscopy score on days 14 and 55. On day 55 Il10−/− mice from both groups displayed an opaque mucosa and altered vascular pattern (Fig. 8a). The thickening of the colon became more prominent, thin faeces were discovered and the diseased regions presented frequently with mucosal bleeding (MEICS 10·0 ± 2·5, n > 5 for mice devoid of ABT-737 compared to 11·1 ± 2·9, n > 5 for mice upon ABT-737, Fig. 8b).
Figure 8.
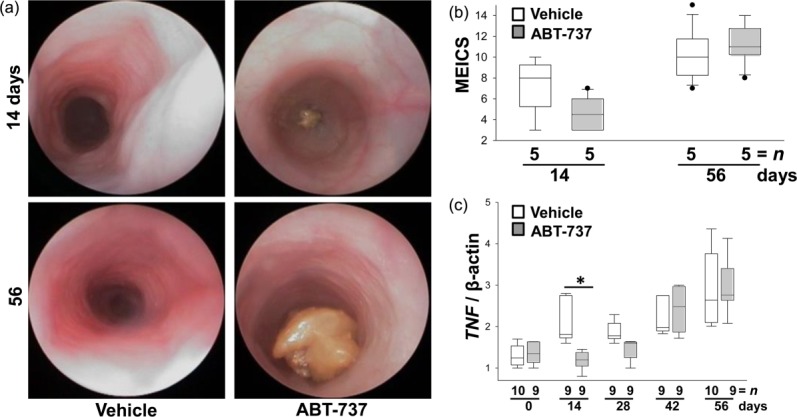
During long-term treatment interleukin (IL)-10−/− mice develop intestinal inflammation despite ABT-737 application (50 mg/kg/3 days for 14 and 56 days), similar to vehicle-receiving mice. (a) Representative images demonstrate colonoscopy pictures derived from ABT-737- and vehicle-treated IL-10−/− mice. (b) Statistical analysis of colonoscopy score [murine endoscopic index of colitis severity (MEICS)]. Colonoscopy was performed on days 14 and 56 (1 day prior to killing the animals). Box-plots express median, 95/5% percentiles and outliers. (c) The cytokine profile of peripheral blood lymphocytes isolated from IL-10−/− is altered in a time-dependent manner upon ABT-737 treatment compared to vehicle controls. Tumour necrosis factor (TNF) mRNA expression levels determined by quantitative polymerase chain reaction (qPCR). Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*).
The cytokine profile in peripheral blood was assessed on days 0, 14, 28, 42 and 56 without further prestimulation of lymphocytes at mRNA level (Fig. 8c). In support of data from the short time-frame experiments mentioned above, TNF gene expression was decreased significantly on day 14 in ABT-737-treated mice compared to vehicle-treated mice. No significant changes were recorded between treatment with ABT-737 and vehicle on day 56.
Discussion
As dysregulated apoptosis of activated lymphocytes driven by increased anti-apoptotic BCL-2 and BCL-XL contributes to the pathogenesis of IBD 3,4,6,7,32, we analysed the potential of the priming agent ABT-737 to limit the persistence of lymphocytes in mouse models of colitis.
In the present study, we applied pro-apoptotic ABT-737 in both the murine model of acute DSS-induced colitis and the Il10−/− model of spontaneous colitis. Following i.p. administration, ABT-737 was detected in peripheral blood, colon tissue and IEC. We determined ABT-737 accumulation in the faeces. ABT-737 treatment led to lymphopenia in mice, demonstrated by the reduced lymphocyte and thrombocyte counts as well as the increased fraction of combined granulocytes and monocytes compared to controls. PBL were cleared in a dose-dependent manner upon ABT-737 and proportions of CD8+ appeared to be diminished. Increased apoptosis upon ABT-737 was determined in PBL, splenocytes and Peyer’s patches and was accompanied by a decrease in TNF and IL1b gene expression compared to vehicle control. CD4+CD44+CD62L+ central memory T cells were decreased in PBL upon ABT-737 compared to vehicle-receiving controls. CD8+ and CD44+CD62L− central memory T cells were also decreased upon ABT-737. Naive T cells require both MCL-1 and BCL-2 for survival (Ivan Dzhagalov et al.) 33. In our own experiments, CD4+ naive T cells are hardly affected by ABT-737, which is not inhibiting MCL-1. This may protect T cell subpopulations from initiation of apoptosis and contribute to a low BIM/BCL-2 ratio in the remaining cells. ABT-737 positively alters the colonic mucosa at both macroscopic and microscopic levels, while also ameliorating intestinal inflammation in models of colitis, as shown by MEICS, histological score and colon length. Inactivation of BCL-2 is key to the ability of BIM to induce apoptosis. BIM can also bind directly to pro-apoptotic BAX and BAK in order to initiate apoptosis 34. Other BH3-only proteins such as BMF, BAD, NOXA and PUMA are considered to act as sensitizers which bind the prosurvival BCL-2 protein and thereby displace BIM from BCL-2 to promote cell death 35. As inhibition of BCL-2 by ABT-737 is mediated by BIM, we also applied ABT-737 to both Il10−/− × Bim−/− upon spontaneous colitis and Bim−/− upon DSS-induced acute colitis and confirmatively mice were found to be relatively resistant to the lymphocyte apoptosis upon ABT-737 administration. In our own experiments we could also show that following 14 days of ABT-737 pretreatment in vivo, gut-homing CD4+CD62L+ cells became more susceptible to apoptosis compared to vehicle controls. BIM/BCL-2 balance is critical for maintaining T cell homeostasis 9. However, the BIM/BCL-2 ratio was decreased with age and during the course of treatment in our own experimental setup. Thus, long-term treatment for a period of 56 days resulted in adapted TNF levels and macroscopic mucosal damage.
ABT-737 affects various transformed cells, while exhibiting minimal toxicity toward normal cells. Navitoclax, the orally administered form of ABT-737, has been applied in chronic lymphocytic leukaemia (CLL). CLL is characterized by the accumulation of lymphocytes, typically of B cell origin, in the blood, lymph nodes and spleen. In a Phase I study of 26 patients, nine achieved a partial response and seven maintained stable disease for more than 6 months 36. Furthermore, the expression of Bim, and the extent of its association with Bcl-2, correlates with in-vivo ABT-737 sensitivity in CLL 27. Navitoclax has also been tested successfully in small cell lung cancer xenograft models. Although navitoclax exhibited an important range of anti-tumour activity, leading to complete tumour regression in several models 37, Bcl-2 targeting by navitoclax showed limited single-agent activity against advanced and recurrent small cell lung cancer in a Phase II study 38.
Studies observing ABT-737 in inflammatory conditions characterized by the uncontrolled proliferation of lymphocytes yielded results which corroborated our own findings. ABT-737 was efficacious in reducing accumulated lymphocytes associated with tissue and organ damage in other animal models of autoimmunity. Collagen-induced arthritis in DBA/J mice is a well-established rodent model for rheumatoid arthritis, which is characterized clinically by paw swelling leading to ankylosis. Treatment with ABT-737 and dexamethasone reduced the mean arthritic score and paw swelling significantly compared with the vehicle-treated group 24. ABT-737 treatment was associated with lymphocyte and platelet depletion. In parallel, ABT-737 was applied in a murine model of delayed-type hypersensitivity (DTH) in C57BL/6 mice. The DTH test is used to determine whether a previous exposure to a specific antigen has occurred. This reaction has been shown to be dependent upon CD4+ and CD8+ memory T cells. Treatment with ABT-737 and dexamethasone showed a similar reduction in paw swelling compared to the vehicle-treated group 24. ABT-737 was also applied in a murine model of interferon (IFN)-γ-induced lupus nephritis. Treatment with ABT-737 prolonged survival and reduced glomerulonephritis to a similar extent to that observed with mycophenolate mofetil 24. In animal models of systemic lupus erythematosus, a decrease in lymphocyte apoptosis is associated clearly with more severe disease pathogenesis. Both Bcl-2-transgenic mice 39 and BIM-deficient mice 40 show evidence of systemic lupus erythematosus-like clinical symptoms caused by prolonged survival of lymphocytes. In patients with lupus, elevated Bcl-2 expression in lymphocytes has been reported 41.
The regulative function of ABT-737 on uncontrolled proliferation of lymphocytes could also be confirmed with a different approach. ABT-737 suppresses allogeneic T and B cell responses after skin transplantation 42. In vitro, ABT-737 prevented allogeneic T cell activation, proliferation and cytotoxicity by inducing apoptosis. The physiological functions of the remaining viable T cells were not impaired. In vivo, ABT-737 was highly selective for lymphoid cells and inhibited allogeneic T and B cell responses after skin transplantation.
In recent years the importance of expansion and contraction of activated lymphocytes by apoptosis has been discussed extensively. Failure in the apoptotic mechanism of lymphocyte control can lead to the development of autoimmunity. Inducing the apoptosis of lymphocytes may provide the basis for a new therapeutic strategy in CD patients. Furthermore, investigating the imbalance between pro-and anti-apoptotic proteins could help to predict clinical relapse upon standard medical therapy. Ultimately, this knowledge could lead to the development of new therapeutic options for the treatment of IBD by focusing on controlling the abnormally large population of activated cells and thereby restoring a physiological turnover of lymphocytes and homeostasis.
Acknowledgments
We thank Professor Dr Thomas Fehr (Division of Nephrology, University Hospital Zurich, Switzerland) for providing ABT-737, murine intestinal tissue and invaluable help with the start-up of the project. This research was supported by grant IBD-0324R from the Broad Medical Research Foundation (BMRF) to M. H. This research was also supported by grant Project no. 2013-16 from the Swiss inflammatory bowel disease cohort study (SIBDCS) to M. H. and by grant Project no. 314730_152895/1 from the Swiss National Science Foundation.
Author contributions
For the study concept: M. H.; for acquisition of the data: C. L., M. M., V. T., M. C., B. L. M., C. L. G. and M. H.; for critical revision of the manuscript: C. L., P. C., B. L. M., C. L. G., M. F. and G. R.
Disclosure
C. L., M. M., V. T., M. C., P. C., M. F. and M. H. have no conflicts of interest to disclose. G. R. discloses grant support from AbbVie, Ardeypharm, MSD, FALK, Flamentera, Novartis, Roche, Tillots, UCB and Zeller. B. L. M. and C. L. G. are employees of AbbVie. B. L. M. and C. L. G. provided ABT-737, performed MS analysis and further contributed to the discussion of the data; however, AbbVie did not provide any additional financial support (e.g. writing/editing of the manuscript).
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher’s Web site:
Fig. S1. Cell death upon ABT-737 is selective for peripheral blood (PBL). (a–c) C57-BL/6J-Fue and Bim−/− mice upon dextran sulphate sodium (DSS)-induced acute colitis (vehicle > white, ABT-737 > grey). Haematological analyses revealed (a) a significantly decreased number of lymphocytes and (b) a significantly increased number of granulocytes upon ABT-737 compared to vehicle controls. Analysis of variance (anova) and Kruskal–Wallis anova on ranks (Dunn’s method). (c) ABT-737 levels were related directly to the removal of lymphocytes.
Fig. S2. Increased apoptosis in the spleen upon ABT-737 treatment. (a–d) C57BL/6 mice with dextran sulphate sodium (DSS)-induced acute colitis. (a) Haematoxylin and eosin (H&E) staining and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells (red) in spleen upon ABT-737 treatment. Mice treated with ABT-737 or vehicle received either (a) water or (b) DSS. Mice were killed at day 9. Treatment with ABT-737 was followed by an increase in TUNEL+ cells in both red pulp and white pulp. Images representative for five mice each. Original magnification as indicated. (c) Spleen weights in DSS-induced acute colitis in C57BL/6 mice. C57BL/6 mice were treated with ABT-737 or vehicle and received either water or DSS. Treatment with ABT-737 was followed by a decrease in spleen weight. Induction of colitis was followed by a significant increase of the spleen, shown to be decreased significantly upon ABT-737. Statistical analysis was performed using the Kruskal–Wallis one way analysis of variance (anova), all pairwise multiple comparison procedures (Dunn’s method), P < 0·05 (*). (d) Spleen of a mouse which received vehicle (left) or ABT-737 (right) upon DSS. Images representative for 14 and 20 mice, respectively.
Fig. S3. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737 compared to vehicle treatment in the IL-10−/− model of spontaneous colitis. Mice upon ABT-737 or vehicle were killed at day 5. Original magnification × 630. Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*). The number of cells was calculated from four high-power fields for each mouse.
Fig. S4. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737. Water control mice upon vehicle or ABT-737. Mice were killed at day 9. Treatment with ABT-737 was followed by an increase in TUNEL+ cells in Peyer’s patches of the small bowel. Images representative for five mice each. Original magnification as indicated.
Fig. S5. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737. Mice on dextran sulphate sodium (DSS) were killed at day 9. Treatment with ABT-737 was followed by an increase in TUNEL+ cells in Peyer’s patches. Images representative for five mice each. Original magnification as indicated.
Fig. S6. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737 treatment in dextran sulphate sodium (DSS)-induced acute colitis in C57BL/6 mice. TUNEL+ cells frequently appeared as clusters of more than four cells in mice treated with ABT-737 compared to vehicle. Original magnification as indicated.
Fig. S7. Mice suffering from colitis develop an ameliorated intestinal inflammation upon ABT-737 treatment compared to vehicle-receiving mice. (a and b) C57BL/6 mice with dextran sulphate sodium (DSS)-induced acute colitis. (a) Representative images demonstrate colonoscopy pictures derived from ABT-737- and vehicle-treated mice. (b) Statistical analysis of colonoscopy score [murine endoscopic index of colitis severity (MEICS)]. Statistical analysis was performed using the Kruskal–Wallis one-way analysis of variance on ranks (Dunn’s method).
Fig. S8. ABT-737 treatment was followed by a significant increase of the colon length. (a,b) Acute dextran sulphate sodium (DSS)-induced colitis in C57BL/6 mice. (a) Mice upon ABT-737 or vehicle received either water or DSS. Statistical analysis was performed using the one-way analysis of variance (anova), all pairwise multiple comparison procedures (Bonferroni). (b) ABT-737 treatment was followed by a decrease in CD3+ in colonic lamina propria; original magnification × 10.
Fig. S9. ABT-737 treatment was followed by a significant increase of apoptosis in CD4+CD62Lv in a dose-dependent manner. Contour plots. Following ABT-737 in vivo pretreatment CD4+CD62L+ cells became susceptible to apoptosis initiated by ABT-737 compared to vehicle controls (ABT-737 concentration as indicated). Living cells, annexin V−/propidium iodide (PI)−; apoptotic cells, annexin V+/PI−.
Fig. S10. Depigmentation of the abdominal fur. Hair depigmentation after 56 days of long-term treatment of IL-10−/− with ABT-737 (50 mg/kg/3 days).
References
- Mitchell T, Kappler J, Marrack P. Bystander virus infection prolongs activated T cell survival. J Immunol. 1999;162:4527–35. [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–67. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Boirivant M, Marini M, Di Felice G, et al. Lamina propria T cells in Crohn’s disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology. 1999;116:557–65. doi: 10.1016/s0016-5085(99)70177-0. [DOI] [PubMed] [Google Scholar]
- Ina K, Itoh J, Fukushima K, et al. Resistance of Crohn’s disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J Immunol. 1999;163:1081–90. [PubMed] [Google Scholar]
- Itoh J, de La Motte C, Strong SA, Levine AD, Fiocchi C, et al. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn’s disease. Gut. 2001;49:35–41. doi: 10.1136/gut.49.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- Iborra M, Moret I, Buso E, et al. Differential regulation of oxidative stress and apoptosis related genes during active and inactive Crohn’s disease (CD) Gastroenterology. 2012;142(Suppl. 1):S–877. [Google Scholar]
- Veltkamp C, Anstaett M, Wahl K, et al. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFalpha treatment. Gut. 2011;60:1345–53. doi: 10.1136/gut.2010.217117. [DOI] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–75. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Tischner D, Woess C, Ottina E, Villunger A, et al. Bcl-2-regulated cell death signalling in the prevention of autoimmunity. Cell Death Dis. 2010;1:e48. doi: 10.1038/cddis.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A, Lindsay JO, Sturm A. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Doering J, Begue B, Lentze MJ, et al. Induction of T lymphocyte apoptosis by sulphasalazine in patients with Crohn’s disease. Gut. 2004;53:1632–8. doi: 10.1136/gut.2003.037911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Meng C, Ivashkiv LB, et al. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci USA. 2000;97:9573–8. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel AW, Mela CM, Lindsay JO, Gazzard BG, Goodier MR. Increased proportion of CD16(+) NK cells in the colonic lamina propria of inflammatory bowel disease patients, but not after azathioprine treatment. Aliment Pharmacol Ther. 2011;33:115–26. doi: 10.1111/j.1365-2036.2010.04499.x. [DOI] [PubMed] [Google Scholar]
- Ben-Horin S, Goldstein I, Fudim E. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58:396–403. doi: 10.1136/gut.2008.157339. [DOI] [PubMed] [Google Scholar]
- Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–45. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut. 2002;50:206–11. doi: 10.1136/gut.50.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology. 2001;121:1145–57. doi: 10.1053/gast.2001.28702. [DOI] [PubMed] [Google Scholar]
- Atreya R, Zimmer M, Bartsch B. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14(+) macrophages. Gastroenterology. 2011;141:2026–38. doi: 10.1053/j.gastro.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Chen S, Dai Y, Pei XY, Grant S, et al. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–69. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell PD, Gu J, McCarthy D. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol. 2009;182:7482–9. doi: 10.4049/jimmunol.0802813. [DOI] [PubMed] [Google Scholar]
- Liu H, Pope RM, et al. The role of apoptosis in rheumatoid arthritis. Curr Opin Pharmacol. 2003;3:317–22. doi: 10.1016/s1471-4892(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Nagy G, Koncz A, Perl A. T- and B-cell abnormalities in systemic lupus erythematosus. Crit Rev Immunol. 2005;25:123–40. doi: 10.1615/critrevimmunol.v25.i2.30. [DOI] [PubMed] [Google Scholar]
- High LM, Szymanska B, Wilczynska-Kalak U. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol. 2010;77:483–94. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- Leucht K, Caj M, Fried M, Rogler G, Hausmann M, et al. Impaired removal of Vbeta8 lymphocytes aggravates colitis in mice deficient for BIM. Clin Exp Immunol. 2013;173:493–501. doi: 10.1111/cei.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Wirtz S. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut. 2005;54:950–4. doi: 10.1136/gut.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W, et al. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–45. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidler L, Hans W, Schotte L. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Kuiper I, Van Duijn W, et al. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- Dzhagalov I, Dunkle A, He YW, et al. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–8. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007;6:2236–40. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Letai A. Displacement of Bim by Bmf and Puma rather than increase in Bim level mediates paclitaxel-induced apoptosis in breast cancer cells. Cell Death Differ. 2010;17:1624–35. doi: 10.1038/cdd.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandik-Nayak L, Nayak S, Sokol C, et al. The origin of anti-nuclear antibodies in bcl-2 transgenic mice. Int Immunol. 2000;12:353–64. doi: 10.1093/intimm/12.3.353. [DOI] [PubMed] [Google Scholar]
- Hughes P, Bouillet P, Strasser A, et al. Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases. Curr Dir Autoimmun. 2006;9:74–94. doi: 10.1159/000090773. [DOI] [PubMed] [Google Scholar]
- Miret C, Font J, Molina R. Bcl-2 oncogene (B cell lymphoma/leukemia-2) levels correlate with systemic lupus erythematosus disease activity. Anticancer Res. 1999;19:3073–6. [PubMed] [Google Scholar]
- Cippa PE, Kraus AK, Edenhofer I, et al. The BH3-mimetic ABT-737 inhibits allogeneic immune responses. Transpl Int. 2011;24:722–32. doi: 10.1111/j.1432-2277.2011.01272.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cell death upon ABT-737 is selective for peripheral blood (PBL). (a–c) C57-BL/6J-Fue and Bim−/− mice upon dextran sulphate sodium (DSS)-induced acute colitis (vehicle > white, ABT-737 > grey). Haematological analyses revealed (a) a significantly decreased number of lymphocytes and (b) a significantly increased number of granulocytes upon ABT-737 compared to vehicle controls. Analysis of variance (anova) and Kruskal–Wallis anova on ranks (Dunn’s method). (c) ABT-737 levels were related directly to the removal of lymphocytes.
Fig. S2. Increased apoptosis in the spleen upon ABT-737 treatment. (a–d) C57BL/6 mice with dextran sulphate sodium (DSS)-induced acute colitis. (a) Haematoxylin and eosin (H&E) staining and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells (red) in spleen upon ABT-737 treatment. Mice treated with ABT-737 or vehicle received either (a) water or (b) DSS. Mice were killed at day 9. Treatment with ABT-737 was followed by an increase in TUNEL+ cells in both red pulp and white pulp. Images representative for five mice each. Original magnification as indicated. (c) Spleen weights in DSS-induced acute colitis in C57BL/6 mice. C57BL/6 mice were treated with ABT-737 or vehicle and received either water or DSS. Treatment with ABT-737 was followed by a decrease in spleen weight. Induction of colitis was followed by a significant increase of the spleen, shown to be decreased significantly upon ABT-737. Statistical analysis was performed using the Kruskal–Wallis one way analysis of variance (anova), all pairwise multiple comparison procedures (Dunn’s method), P < 0·05 (*). (d) Spleen of a mouse which received vehicle (left) or ABT-737 (right) upon DSS. Images representative for 14 and 20 mice, respectively.
Fig. S3. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737 compared to vehicle treatment in the IL-10−/− model of spontaneous colitis. Mice upon ABT-737 or vehicle were killed at day 5. Original magnification × 630. Statistical analysis was performed using the Mann–Whitney rank-sum test, P < 0·05 (*). The number of cells was calculated from four high-power fields for each mouse.
Fig. S4. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737. Water control mice upon vehicle or ABT-737. Mice were killed at day 9. Treatment with ABT-737 was followed by an increase in TUNEL+ cells in Peyer’s patches of the small bowel. Images representative for five mice each. Original magnification as indicated.
Fig. S5. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737. Mice on dextran sulphate sodium (DSS) were killed at day 9. Treatment with ABT-737 was followed by an increase in TUNEL+ cells in Peyer’s patches. Images representative for five mice each. Original magnification as indicated.
Fig. S6. Increased number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)+ cells in Peyer’s patches of the small bowel upon ABT-737 treatment in dextran sulphate sodium (DSS)-induced acute colitis in C57BL/6 mice. TUNEL+ cells frequently appeared as clusters of more than four cells in mice treated with ABT-737 compared to vehicle. Original magnification as indicated.
Fig. S7. Mice suffering from colitis develop an ameliorated intestinal inflammation upon ABT-737 treatment compared to vehicle-receiving mice. (a and b) C57BL/6 mice with dextran sulphate sodium (DSS)-induced acute colitis. (a) Representative images demonstrate colonoscopy pictures derived from ABT-737- and vehicle-treated mice. (b) Statistical analysis of colonoscopy score [murine endoscopic index of colitis severity (MEICS)]. Statistical analysis was performed using the Kruskal–Wallis one-way analysis of variance on ranks (Dunn’s method).
Fig. S8. ABT-737 treatment was followed by a significant increase of the colon length. (a,b) Acute dextran sulphate sodium (DSS)-induced colitis in C57BL/6 mice. (a) Mice upon ABT-737 or vehicle received either water or DSS. Statistical analysis was performed using the one-way analysis of variance (anova), all pairwise multiple comparison procedures (Bonferroni). (b) ABT-737 treatment was followed by a decrease in CD3+ in colonic lamina propria; original magnification × 10.
Fig. S9. ABT-737 treatment was followed by a significant increase of apoptosis in CD4+CD62Lv in a dose-dependent manner. Contour plots. Following ABT-737 in vivo pretreatment CD4+CD62L+ cells became susceptible to apoptosis initiated by ABT-737 compared to vehicle controls (ABT-737 concentration as indicated). Living cells, annexin V−/propidium iodide (PI)−; apoptotic cells, annexin V+/PI−.
Fig. S10. Depigmentation of the abdominal fur. Hair depigmentation after 56 days of long-term treatment of IL-10−/− with ABT-737 (50 mg/kg/3 days).