Figure 9.
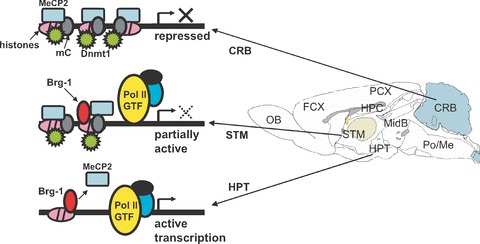
A proposed molecular mechanism for MOR gene regulation. MeCP2 and Dnmt1 bind to hypermethylated DNA form a histone-associated repressor complex, silencing the MOR gene in cerebellar tissue. In the cerebellum, hypermethylation of CpGs around the proximal promoter (PP) coincides with increased interactions with MeCP2 and Dnmt1. This might lead to compaction of the chromatin structure after histone modifications, followed by silencing of the MOR gene in these cells. In striatal cells, intermediate methylation of CpGs around the PP begins as MeCP2 start to dissociate and Brg1 is recruited, concurrent with histone modifications, resulting in intermediate levels of MOR expression. In the hypothalamus, nearly complete demethylation of the CpGs around the PP is observed as MeCP2 dissociates and Brg1 is recruited. Hyperacetylation of histones also occur in the promoter, suggesting active transcription of the MOR gene in the hypothalamic tissue. The components for active transcription shown in the figure, i.e. GTF (general transcription factors) and their associated-RNA polymerase II (Pol II), are putative factors for general genes.
