Abstract
Objective
To examine the association of menarche timing with cardiometabolic risk factors into early to mid-adulthood, comparing African-American and White women.
Study design
Analyses included 2,583 women (African-American=1,333; White=1,250) from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort study over 25 years of follow-up (1985–2011). Outcomes included type 2 diabetes, metabolic syndrome, adiposity, glucose, insulin, blood pressure, and blood lipids. Cox models or repeated measures linear regression models estimated the association between age at menarche and the outcomes.
Results
Each one-year earlier age at menarche was associated with higher mean BMI among African-American (0.88±0.12 kg/m2, p<.0001) and White (0.89±0.10 kg/m2, p<.0001) women. After BMI adjustment, each one-year earlier age at menarche was associated with higher triglycerides (2.26±0.68 mg/dl, p=0.001) and glucose (0.34±0.11 mg/dl, p=0.002), and greater risk for incident impaired fasting glucose (HR=1.13 95% CI 1.04–1.20) and metabolic syndrome (HR=1.19, 95% CI 1.11–1.26) among white women only.
Conclusions
Excess adiposity associated with earlier menarche is sustained through mid-adulthood, and primarily drives higher cardiometabolic risk factor levels. However, White women with earlier menarche had increased risk of a number of insulin-resistance related conditions independent of adiposity. The cardiometabolic impact of earlier menarche was weaker in African-American women despite higher average adiposity. Weight maintenance would likely reduce, but may not completely eliminate the elevated cardiometabolic risk of earlier menarche.
Keywords: Epidemiology, Menarche, Puberty, Type 2 diabetes, Metabolic Syndrome, Obesity
A growing number of studies report associations between early age at menarche and increased risk for cardiovascular disease (CVD)-related risk factors, such as type 2 diabetes (T2DM), high blood pressure, and metabolic syndrome (MetS) [1–7]. However, early menarche is also strongly associated with obesity, which increases the risk for these adulthood diseases [8, 9]. Recent studies conflict about the confounding or mediating role of childhood and adulthood obesity in the relationship between earlier puberty and cardiometabolic risk factors [1–3, 6, 10, 11], possibly because childhood obesity and puberty may influence each other through common pathways such as hormonal changes and insulin resistance [5, 12]. Longitudinal data on this topic are scarce [5] and would help clarify the relationship between the timing of menarche, adiposity accumulation, and other cardiometabolic risk factors.
Although race appears to be an independent risk factor for early menarche [13, 14], as well as for certain cardiometabolic conditions [15], the relationship of age at menarche with cardiometabolic risk among African-American women has not been well studied. Most reports have included women of European descent only [6, 10, 11, 16–20] or did not look at risk specifically among African-American women [2, 21, 22]. In the Atherosclerosis Risk in Communities (ARIC) study, age at menarche was associated with T2DM among White, but not African-American women during mid to late-adulthood [3].
The current study evaluates the association between age at menarche and CVD risk factors in a prospective cohort of young to middle-aged women with the following aims: (1) assess race-specific associations between age at menarche and incident T2DM, impaired fasting glucose (IFG), and MetS over 25 years of follow up; and (2) examine associations of age at menarche with components of cardiometabolic risk (adiposity, glucose, insulin, blood pressure, and blood lipids) during follow up. We also considered whether any observed associations were independent of BMI measured in young adulthood. We hypothesized an independent association of earlier age at menarche with cardiometabolic risk factors in adulthood, and given findings from ARIC, stronger associations among White women.
Methods
We used data from the Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA included 5,115 African-American and White men (n=2,328) and women (n=2,787) aged 18–30 years at baseline and was designed to study the role of lifestyle and the evolution of CVD risk factors in young adults. The details of the study cohort, including eligibility criteria, sources and methods of recruitment and follow up have been described in detail elsewhere [23]. Briefly, participants were recruited from four US communities: Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Data for our analyses were collected during clinic visits at the baseline and follow-up exams over 25 years (1985–2011). The retention rates at years 2, 5, 7, 10, 15, 20, and 25 were 90%, 86%, 81%, 79%, 74%, 72%, and 72%, respectively. The institutional review boards at each of the study sites approved the study, and participants provided informed consent.
Analyses excluded women with missing age at menarche (n=118), or who reported their age at menarche as <8 (n=1) or >17 (n=9) years as we are interested in studying women within the normal range of menarche timing. Other exclusions were those with diabetes (n=20), missing diabetes status (n=45), or missing BMI (n=11) at the baseline visit, for an overall sample size of 2,583 women (African-American=1,333, White=1,250). For incident IFG and MetS, women with those conditions at baseline were also excluded (IFG: n=38; MetS: n=49) to ensure that onset was after completion of puberty (i.e. incident cases instead of prevalent cases). The final sample for the IFG and MetS analyses were 2,545 and 2,534, respectively. For the analysis of changes in cardiometabolic risk factors during follow up, time points were excluded for patients who were pregnant or missing an outcome variable, or who had developed diabetes at any time prior to the visit. The baseline demographic and cardiovascular risk factor characteristics of those included in these analyses were similar to those of the overall cohort before exclusions [23].
Age at menarche was defined as the age in whole years at the first menstrual period. Age at menarche was assessed at baseline and at visit 2 via self-report on a questionnaire by asking “How old where you when you began menstruating?”. Correlation between reported age at menarche at baseline and at visit 2 was high (r=0.84). We used age at menarche reported at the baseline visit because it was closest in time to the event. We included age at menarche reported at baseline as both continuous and categorical (early [8– 11 years], average [12– 13 years], and late [14– 17 years]) variables in separate models.
Diabetes status was assessed at baseline and at each CARDIA clinic visit. T2DM was defined among non-pregnant women as fasting (for 8 hours) glucose≥7.0 mmol/l (126 mg/dl), HbA1c≥6.5%, 2-hour oral glucose tolerance≥11.1 mmol/l (200 mg/dl), or use of diabetes medication. The 2-hour oral glucose tolerance test was administered at visit years 10, 20, and 25 only, and HbA1c was measured at years 20 and 25 only.
IFG was defined as fasting glucose ≥5.6 mmol/l (100 mg/dl) but <7.0 mmol/l (126 mg/dl) and not taking diabetes medication. MetS was defined according to the National Cholesterol Education Program's Adult Treatment Panel III report (NCEP ATP-III) revised criteria for women of 3 or more of the following factors: waist circumference>88 cm, systolic blood pressure≥130/85 mmHg (or use of anti-hypertensive medication), HDL-C<2.8 mmol/l (50 mg/dL), triglycerides≥8.3 mmol/l (150 mg/dL) (or use of lipid-lowering medication), or fasting glucose≥5.6 mmol/l (100 mg/dl) (or use of hypoglycemic medication) [24].
Blood pressure was measured three times at each visit after a five-minute rest, and the mean of the last two measurements were used in this analysis. Weight (kg), height (cm), and waist circumference (cm) were measured at each clinic visit while participants dressed in scrub suits and removed shoes. Body height (cm) and weight (kg) were measured with a calibrated scale and a vertical ruler. BMI was calculated as weight (kg)/height2 (m2) at each exam. An enzymatic method was used to measure plasma concentrations of total cholesterol, HDL cholesterol, and triglycerides. HDL cholesterol was measured after dextran-magnesium precipitation [25], and LDL cholesterol was calculated by using the Friedewald equation [26]. The test-retest reliability coefficients for split specimens of total, HDL cholesterol, LDL cholesterol, and triglycerides were high, at >0.98 [27]. Additional details of the CARDIA examination procedures were published previously [27, 28].
The covariates age (years), race (African American or White), parental history of diabetes (yes/no), oral contraceptive use (yes/no), smoking status (never, former, current), physical activity level (MET-hours/week), alcohol consumption (g/day), and education (<high school, high school, >high school) were all self-reported at baseline. Physical activity prior to high school and during high school was recalled at the baseline visit using an activity scale of 1 (physically inactive) to 5 (very active). Antihypertensive and hypoglycemic medication use was queried at all visits, and use of lipid-lowering medications at year 2. Menopause status (yes/no) was obtained by self report at visit years 15, 20, and 25.
Statistical analyses
Baseline participant characteristics by race and age at menarche category were summarized using mean (SD) for continuous variables or n (%) for categorical variables. The linear trend was tested using the likelihood ratio test by modeling the value in each menarche category as an ordinal variable. All outcome variables were assessed for normality. Triglycerides and insulin were skewed and therefore natural log transformed for analyses. There were no qualitative differences between the associations for transformed and non-transformed values, so non-transformed data are presented for simplicity.
Cox proportional hazards models were used to estimate hazard ratios (HR) for incident T2DM (yes/no), IFG (yes/no), or MetS (yes/no) according to continuous age at menarche. We first included age at menarche as a categorical (8–11, 12–13, and 14–17 years) variable in models, and then as a continuous variable after ensuring that associations were not curvilinear. Estimates (HR and 95% CI) were calculated for all women, and stratified by race as our a priori research question was about race differences in associations. The proportional hazards assumption was tested and verified by generating a time-dependent predictor and creating interactions of age at menarche and a log function of survival time, and then the Wald chi-square procedure tested whether the coefficients were equal to 0.
Separate repeated measures linear regression models accounting for the correlated nature of the data were used to examine the association of age at menarche with each cardiometabolic risk factor (BMI, waist circumference, total cholesterol, triglycerides, LDL-C, HDL-C, blood pressure, insulin, and glucose) over 25 years of follow up. All models included the independent variables continuous age at menarche, follow-up year (the time variable), age and age2, and clinical center, and assumed a compound symmetry correlation matrix. The cardiometabolic risk factors were dependent continuous variables in each of the separate models. Models were run in a series by adding covariates, starting with minimally adjusted Model 1 (age, age2, center, and time). Model 2 also included variables that were associated with age at menarche and/or cardiometabolic risk factors in prior studies: race (other than for race-stratified models), alcohol use, education, smoking status, physical activity prior to high school, physical activity during high school, baseline physical activity, parental history of diabetes, oral contraceptive use, and menopause status. Blood pressure models additionally adjusted for use of antihypertensive medication use (yes/no) and lipid models for cholesterol-lowering medications use (yes/no). Finally, to test whether associations were independent of adiposity, the models were further adjusted by the addition of baseline (age 18–30 years) BMI (kg/m2) in Model 3. Most models indicated linear, dose-response effects of age at menarche. Therefore, age at menarche was included as continuous variable in the repeated regression models, except for display of adjusted means for each menarche category (early, average, late) for BMI and waist circumference at each CARDIA visit in the Figure. To test for differences by race, adjusted models were run with an interaction term (race*menarche age).
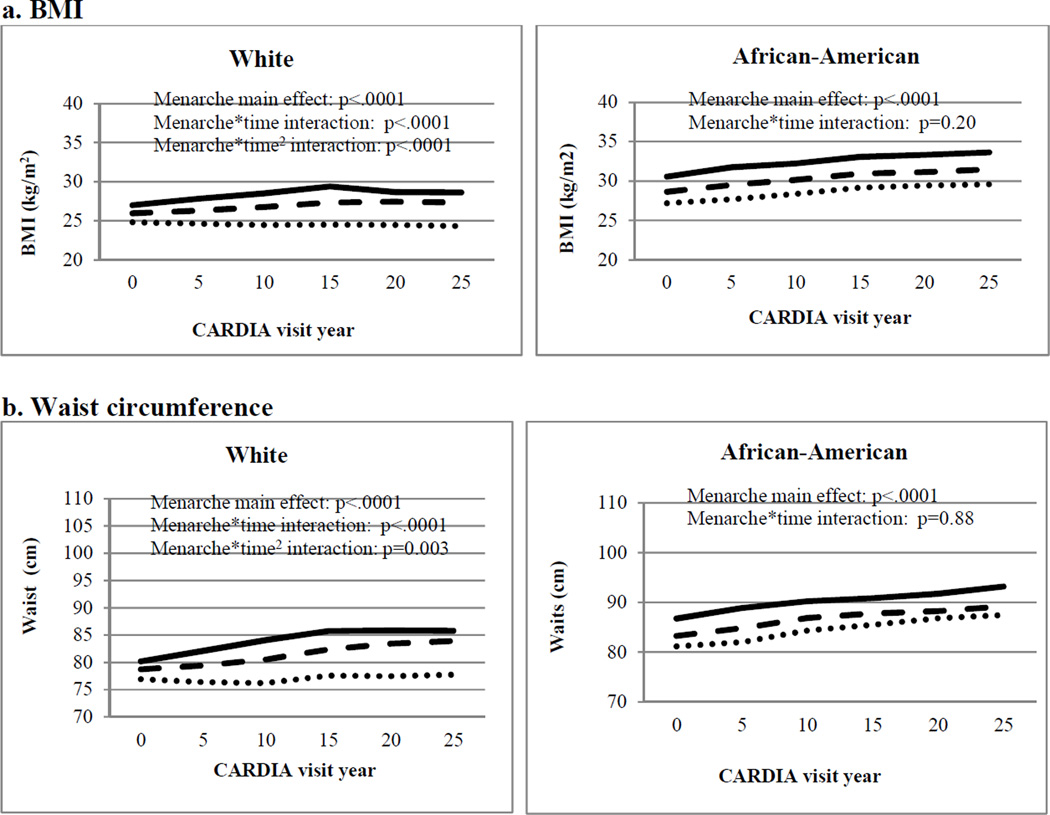
Figure 1.
Adjusted* mean adiposity levels during adulthood by age at menarche category in the CARDIA Study
Legend:
Early  Average
Average  Late
Late 
Notes:
Includes women who reported menarche 8–17 years, were non-diabetic at baseline, and who had data available on covariates. Excludes the visits for women who were pregnant or developed diabetes.
*Adjusted for age, age2, and clinical center
All analyses were run in SAS version 9.2. The alpha statistical significance level was set at p<0.05 for the Cox proportional hazard models and interactions. Bonferroni corrected p-values <0.005 (p=0.05/10 outcomes) were considered to be statistically significant for the purposes of the longitudinal analyses to adjust for multiple comparisons.
Results
After exclusions, the overall cohort of 2,583 women had a mean age of 25 years at baseline (range=18–30 years) and 50 years at the final visit (range=42–59 years). At the year 25 visit, our overall cohort included 1,849 women. The mean age at menarche was 12.6 years (SD=1.5) for the total sample, and was younger for African-American (12.5 years, SD=1.5) compared with White (12.7 years, SD=1.5) women (t=−3.66, p-value<.001).
Table I shows the unadjusted baseline characteristics of the study sample by age at menarche category. For both African-American and White women, earlier menarche was associated with lower high school physical activity level, as well as greater BMI, waist circumference, and insulin at baseline. Baseline glucose and systolic blood pressure were higher, and physical activity level prior to high school was lower among earlier maturing African-American women. Shorter stature, less alcohol consumption, greater parental history of diabetes, and lower HDL-C were more frequent among White women with early menarche.
Table 1.
Baseline characteristics of the study population by age at menarche category: the CARDIA Study
| Age at Menarche (years) | ||||
| 8–11 | 12–13 | 14–17 | ||
| African-American (n=1,333) | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | p-trend |
| n (%) | 294 (22.1) | 749 (56.2) | 290 (21.8) | |
| Age at menarche (years) | 10.5 (0.8) | 12.5 (0.5) | 14.7 (0.8) | |
| Age at baseline (years) | 24.3 (3.8) | 24.6 (3.8) | 23.9 (4.0) | 0.35 |
| BMI (kg/m2) | 27.6 (6.6) | 25.8 (6.4) | 24.0 (5.7) | <.0001 |
| Waist circumference (cm) | 79.4 (13.7) | 76.4 (13.1) | 73.5 (11.7) | <.0001 |
| Height (cm) | 163.0 (7.0) | 163.8 (6.5) | 163.8 (7.3) | 0.14 |
| Physical activity at baseline (MET h/week) | 283.4 (206.5) | 270.3 (234.2) | 294.1 (231.5) | 0.60 |
| Physical activity prior to high school (activity index) | 3.5 (1.2) | 3.7 (1.2) | 3.9 (1.2) | 0.0005 |
| Physical activity during high school (activity index) | 3.7 (1.2) | 3.9 (1.1) | 3.9 (1.1) | 0.01 |
| Smoking status | 0.97 | |||
| Never | 177 (60.2) | 461 (61.6) | 173 (59.9) | |
| Former | 25 (8.5) | 67 (9.0) | 26 (9.0) | |
| Current | 92 (31.3) | 221 (29.5) | 90 (31.1) | |
| Alcohol (g/day) | 5.1 (11.1) | 4.9 (10.7) | 6.5 (16.5) | 0.15 |
| Ever used oral contraceptives (yes) | 240 (82.0) | 590 (78.8) | 225 (77.6) | 0.20 |
| Education | 0.21 | |||
| <High school | 27 (9.2) | 70 (9.3) | 28 (9.7) | |
| High school | 200 (68.0) | 537 (71.6) | 209 (72.1) | |
| >High school | 67 (22.8) | 143 (19.1) | 53 (18.2) | |
| Parental history of diabetes (yes) | 83 (28.1) | 186 (24.7) | 63 (21.7) | 0.07 |
| Impaired fasting glucose (yes) | 4 (1.4) | 16 (2.1) | 3 (1.0) | 0.77 |
| Glucose (mg/dl) | 80.3 (7.5) | 79.3 (8.5) | 79.0 (8.0) | 0.05 |
| Insulin (uU/ml) | 14.6 (7.6) | 13.0 (6.3) | 12.8 (5.5) | 0.004 |
| LDL-C (mg/dl) | 110.0 (31.8) | 110.9 (31.5) | 110.4 (33.0) | 0.87 |
| HDL-C (mg/dl) | 54.8 (13.4) | 55.4 (12.7) | 55.7 (13.0) | 0.33 |
| Triglycerides (mg/dl) | 62.8 (30.5) | 63.1 (30.8) | 63.2 (37.6) | 0.92 |
| Total cholesterol (md/dl) | 177.3 (34.7) | 178.9 (33.3) | 178.7 (34.8) | 0.58 |
| Systolic blood pressure (mmHg) | 109.0 (10.3) | 108.4 (10.1) | 106.6 (9,1) | 0.003 |
| Diastolic blood pressure (mmHg) | 68.2 (9.6) | 67.5 (9,3) | 66.6 (9.4) | 0.03 |
| Age at Menarche (years) | ||||
| 8–11 | 12–13 | 14–17 | ||
| White (n=1,250) | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | p-trend |
| n (%) | 213 (17.0) | 736 (58.9) | 301 (24.1) | |
| Age at menarche (years) | 10.6 (0.7) | 12.5 (0.5) | 14.7 (0.9) | |
| Age at baseline (years) | 25.4 (3.6) | 25.5 (3.4) | 25.4 (3.3) | 0.99 |
| BMI (kg/m2) | 24.4 (5.4) | 23.1 (4.3) | 22.0 (3.3) | <.0001 |
| Waist circumference (cm) | 73.8 (11.1) | 71.9 (9.1) | 70.0 (7.4) | <.0001 |
| Height (cm) | 164.0 (6.2) | 165.4 (6.4) | 165.4 (6.5) | 0.02 |
| Physical activity (MET-h/week) | 385.9 (237.9) | 395.8 (261.8) | 417.9 (271.2) | 0.15 |
| Physical activity prior to high school | 3.5 (1.1) | 3.6 (1.1) | 3.7 (1.1) | 0.19 |
| Physical activity in high school | 3.5 (1.1) | 3.6 (1.1) | 3.8 (1.0) | 0.0001 |
| Current smoker | 0.35 | |||
| Never | 126 (59.2) | 378 (51.4) | 162 (53.8) | |
| Former | 37 (17.5) | 160 (21.7) | 55 (18.3) | |
| Current | 50 (23.5) | 198 (26.9) | 84 (27.9) | |
| Alcohol (g/day) | 6.7 (12.3) | 10.2 (4.6) | 10.5 (17.1) | 0.01 |
| Ever used oral contraceptives | 158 (74.2) | 560 (75.6) | 218 (72.4) | 0.55 |
| Education | 0.90 | |||
| <High school | 10 (4.7) | 27 (3.7) | 14 (4.7) | |
| High school | 112 (52.2) | 344 (46.7) | 152 (50.5) | |
| >High school | 91 (43.0) | 365 (49.6) | 135 (44.9) | |
| Parental history of diabetes (yes) | 38 (17.8) | 112 (15.1) | 35 (11.6) | 0.05 |
| Impaired fasting glucose (yes) | 6 (2.8) | 10 (1.4) | 3 (1.0) | 0.12 |
| Glucose (mg/dl) | 81.4 (8.1) | 80.6 (7.4) | 80.2 (7.0) | 0.08 |
| Insulin (uU/ml) | 11.1 (5.6) | 10.2 (4.6) | 10.1 (4.6) | 0.04 |
| LDL-C (mg/dl) | 106.0 (29.7) | 106.6 (29.2) | 104.0 (27.7) | 0.41 |
| HDL-C (mg/dl) | 55.0 (13.4) | 55.7 (12.8) | 58.3 (13.1) | 0.002 |
| Triglycerides (mg/dl) | 71.9 (36.0) | 69.2 (39.4) | 67.4 (35.4) | 0.19 |
| Total cholesterol (md/dl) | 175.3 (31.2) | 176.1 (31.3) | 175.8 (30.4) | 0.84 |
| Systolic blood pressure (mmHg) | 105.1 (9.3) | 105.1 (9.4) | 104.2 (9,2) | 0.24 0.26 |
| Diastolic blood pressure (mmHg) | 66.9 (8.1) | 66.1 (8.7) | 65.9 (8.2) | 0.26 |
We identified 271 incident cases of T2DM (n=193 African-American, n=78 White) over 25 years of follow up (Table II). Each one-year earlier age at menarche was associated with greater risk for T2DM in Model 1 among White women, but results were not statistically significant after further adjustment for confounders and potential mediation by BMI (Models 2 and 3, respectively). Although HRs were greater for each younger age at menarche among African-American women, the results did not reach statistical significance in any of the models.
Table 2.
Adjusted HRs for younger age at menarche for incident type 2 diabetes, impaired fasting glucose, and metabolic syndrome: The CARDIA Study
| Age at Menarche | |||||
| 8–11 yrs | 12–13 yrs | 14–17 yrs | Continuous (per yr younger) | ||
| HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
p-race interaction |
|
| All Women | |||||
| Type 2 Diabetes | N=69 | N=159 | N=43 | N=271 | |
| Model 1 | 1.69 (1.16, 2.29) | 1.38 (0.99, 1.94) | REF | 1.10 (1.02, 1.17) | 0.20 |
| Model 2 | 1.61 (1.09, 2.37) | 1.35 (0.96, 1.89) | REF | 1.08 (1.00, 1.16) | 0.31 |
| Model 3 | 1.33 (0.90, 1.96) | 1.24 (0.88, 1.75) | REF | 1.04 (0.95, 1.12) | 0.37 |
| IFG | N=146 | N=355 | N=112 | N=613 | |
| Model 1 | 1.49 (1.16, 1.91) | 1.29 (1.05, 1.60) | REF | 1.11 (1.07, 1.16) | 0.08 |
| Model 2 | 1.50 (1.17, 1.94) | 1.31 (1.06, 1.62) | REF | 1.11 (1.06, 1.16) | 1.17 |
| Model 3 | 1.28 (0.99, 1.62) | 1.22 (0.98, 1.51) | REF | 1.08 (1.02, 1.13) | 0.05 |
| MetS | N=158 | N=368 | N=103 | N=629 | |
| Model 1 | 1.96 (1.54, 2.48) | 1.50 (1.22, 1.85) | REF | 1.15 (1.10, 1.20) | 0.0002 |
| Model 2 | 1.95 (1.53, 2.47) | 1.49 (1.20, 1.84) | REF | 1.15 (1.10, 1.19) | 0.0005 |
| Model 3 | 1.45 (1.14, 1.85) | 1.31 (1.06, 1.62) | REF | 1.09 (1.04, 1.14) | <.0001 |
| African-American Women | |||||
| Type 2 Diabetes | N=48 | N=114 | N=31 | N=193 | |
| Model 1 | 1.50 (0.96, 2.36) | 1.33 (0.90, 1.98) | REF | 1.07 (0.97, 1.15) | |
| Model 2 | 1.47 (0.93, 2.33) | 1.29 (0.87, 1.93) | REF | 1.06 (0.96, 1.15) | |
| Model 3 | 1.28 (0.80, 2.04) | 1.28 (0.86, 1.92) | REF | 1.03 (0.92, 1.12) | |
| IFG | N=96 | N=182 | N=70 | N=348 | |
| Model 1 | 1.30 (0.96, 1.77) | 1.03 (0.78, 1.35) | REF | 1.08 (1.01, 1.14) | |
| Model 2 | 1.29 (0.94, 1.77) | 1.02 (0.77, 1.34) | REF | 1.07 (1.01, 1.14) | |
| Model 3 | 1.12 (0.81, 1.54) | 0.97 (0.73, 1.28) | REF | 1.04 (0.97, 1.11) | |
| MetS | N=101 | N=212 | N=75 | N=388 | |
| Model 1 | 1.58 (1.18, 2.09) | 1.23 (0.95, 1.58) | REF | 1.09 (1.03, 1.14) | |
| Model 2 | 1.55 (1.17, 2.08) | 1.20 (0.93, 1.55) | REF | 1.09 (1.03, 1.15) | |
| Model 3 | 1.17 (0.87, 1.57) | 1.07 (0.83, 1.38) | REF | 1.03 (0.97, 1.09) | |
| White women | |||||
| Type 2 Diabetes | N=21 | N=45 | N=12 | N=78 | |
| Model 1 | 2.31 (1.13, 4.71) | 1.48 (0.78, 2.81) | REF | 1.17 (1.03, 1.29) | |
| Model 2 | 1.98 (0.96, 4.13) | 1.41 (0.74, 2.70) | REF | 1.12 (0.97, 1.24) | |
| Model 3 | 1.14 (0.53, 2.46) | 1.00 (0.51, 1.96) | REF | 1.00 (0.82, 1.15) | |
| IFG | N=50 | N=173 | N=42 | N=265 | |
| Model 1 | 1.79 (1.18, 2.69) | 1.75 (1.25, 2.46) | REF | 1.16 (1.09, 1.23) | |
| Model 2 | 1.84 (1.21, 2.80) | 1.89 (1.21, 2.66) | REF | 1.16 (1.08, 1.23) | |
| Model 3 | 1.52 (0.99, 2.33) | 1.63 (1.16, 2.31) | REF | 1.13 (1.04, 1.20) | |
| MetS | N=57 | N=156 | N=28 | N=241 | |
| Model 1 | 3.04 (1.97, 4.69) | 2.26 (1.53, 3.32) | REF | 1.26 (1.19, 1.32) | |
| Model 2 | 3.04 (1.96, 4.72) | 2.35 (1.59, 3.46) | REF | 1.25 (1.18, 1.32) | |
| Model 3 | 2.15 (1.36, 3.39) | 2.02 (1.37, 2.98) | REF | 1.19 (1.11, 1.26) | |
Notes:
Model 1 = age, center, and race adjusted.
Model 2 = Model 1+parental history of diabetes (Y/N), educational status (<HS, HS, >HS), pre-high school physical activity, high school physical activity, smoking status (current, former, never), oral contraceptive use (yes/no), physical activity level (METS), and alcohol intake (g/day).
Model 3 = Model 2+baseline BMI.
Diabetes models exclude women with diabetes at baseline.
IFG models exclude women with diabetes or IFG at baseline.
Metabolic syndrome models exclude women with diabetes or metabolic syndrome at baseline.
There were a total of 613 incident cases of IFG over 25 years of follow up (n= 348 African American, n=265 White) (Table II). Age at menarche was inversely associated with risk for IFG among all women in Models 1 and 2, and remained statistically significantly associated after adjustment for potential mediation by baseline BMI in White women only (Model 3). The association of age at menarche and IFG was stronger for White compared with African-American women after adjusting for baseline BMI (Model 3 p-interaction=0.05)
A total of 629 incident cases of MetS were identified over 25 years of follow up (n=388 African American, n=241 White) (Table II). Age at menarche was inversely associated with risk of MetS, and associations were stronger among White compared with African-American women (Model 3 p-interaction<.0001). The risk for MetS for early vs. late age at menarche was approximately two times greater in white compared with African-American women (White: Model 2 HR=3.04 and Model 3 HR= 2.15; African-American: Model 2 HR=1.55 and Model 3 HR=1.17). Associations were independent of BMI for White but not African-American women. A sensitivity analysis that adjusted for baseline waist circumference, a component of MetS, instead of baseline BMI in Model 3 did not materially change results per one-year younger age at menarche (White: HR=1.20, 95% CI 1.12–1.27; African American: HR=1.05, 95% CI 0.98–1.10).
After exclusions, there were 16,078 observations for the 2,583 women included in the longitudinal analyses, with a mean of 6.2 visits per participant. As shown in the Figure and Table III (available at www.jpeds.com) women with earlier menarche had greater overall mean age-adjusted BMI and waist circumference throughout the 25-year follow-up. Earlier menarche was associated with steeper increases in age-adjusted BMI and waist circumference for White women only (“menarche*time” in the Figure and Table III), and the slopes indicated a strengthening of the association between early menarche and adiposity over time. There were essentially no changes in adjusted mean waist circumference or BMI over the study period for White women with late menarche, but those with early menarche continued to accumulate adiposity until leveling off around visit year 15 (mean age 41 years). Earlier menarche did not appear to influence the slope in adiposity measures for African-American women (p-interaction race<.0001).
Table 3.
Main effecta and per year change in cardiometabolic risk factors over 25 years according to each 1-year earlier age at menarche in CARDIA, age and center adjusted models.
| All women N=2,583 |
African-American N=1,333 |
White N=1,250 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | se | p- value |
Beta | Se | p- value |
Beta | Se | p- value |
p-race interaction |
|
| BMI (kg/m2) | ||||||||||
| Menarche main effect | 0.88 | 0.08 | <.0001 | 0.88 | 0.12 | <.0001 | 0.89 | 0.10 | <.0001 | 0.99 |
| Menarche*time | 0.02 | 0.002 | <.0001 | 0.004 | 0.003 | 0.18 | 0.03 | 0.002 | <.0001 | <.0001 |
| Waist (cm) | ||||||||||
| Menarche main effect | 1.52 | 0.16 | <.0001 | 1.44 | 0.23 | <.0001 | 1.60 | 0.21 | <.0001 | 0.68 |
| Menarche*time | 0.04 | 0.004 | <.0001 | 0.01 | 0.01 | 0.27 | 0.06 | 0.01 | <.0001 | <.0001 |
| Total cholesterol (mg/dl) | ||||||||||
| Menarche main effect | 0.04 | 0.35 | 0.90 | −0.48 | 0.52 | 0.35 | 0.58 | 0.50 | 0.25 | 0.13 |
| Menarche*time | −0.01 | 0.01 | 0.43 | −0.02 | 0.02 | 0.28 | 0.01 | 0.02 | 0.81 | 0.34 |
| LDL-C (mg/dl) | ||||||||||
| Menarche main effect | 0.34 | 0.34 | 0.32 | −0.25 | 0.49 | 0.61 | 0.94 | 0.48 | 0.05 | 0.07 |
| Menarche*time | −0.02 | 0.01 | 0.14 | −0.04 | 0.02 | 0.02 | 0.00 | 0.02 | 0.80 | 0.06 |
| HDL-C (mg/dl) | ||||||||||
| Menarche main effect | −0.72 | 0.16 | <.0001 | −0.35 | 0.21 | 0.10 | −1.09 | 0.24 | <.0001 | 0.02 |
| Menarche*time | −0.02 | 0.01 | 0.0003 | 0.004 | 0.01 | 0.62 | −0.04 | 0.01 | <.0001 | <.0001 |
| Triglycerides (mg/dl) | ||||||||||
| Menarche main effect | 2.12 | 0.45 | <.0001 | 0.56 | 0.58 | 0.34 | 3.71 | 0.70 | <.0001 | 0.0003 |
| Menarche*time | 0.90 | 0.50 | <.0001 | 0.07 | 0.03 | 0.02 | 0.20 | 0.03 | <.0001 | 0.007 |
| Systolic blood pressure (mmHg) | ||||||||||
| Menarche main effect | 0.42 | 0.12 | 0.001 | 0.36 | 0.18 | 0.05 | 0.49 | 0.16 | 0.002 | 0.57 |
| Menarche*time | 0.01 | 0.01 | 0.07 | −0.02 | 0.01 | 0.05 | 0.02 | 0.01 | 0.002 | 0.0005 |
| Diastolic blood pressure (mmHg) | ||||||||||
| Menarche main effect | 0.37 | 0.09 | 0.0001 | 0.31 | 0.14 | 0.03 | 0.46 | 0.13 | 0.0004 | 0.43 |
| Menarche*time | 0.02 | 0.01 | 0.0005 | −0.01 | 0.01 | 0.18 | 0.04 | 0.01 | <.0001 | <.0001 |
| Insulin (μ U/ml) | ||||||||||
| Menarche main effect | 0.35 | 0.06 | <.0001 | 0.29 | 0.10 | 0.003 | 0.43 | 0.07 | <.0001 | 0.26 |
| Menarche*time | 0.02 | 0.003 | <.0001 | 0.001 | 0.01 | 0.89 | 0.03 | 0.002 | <.0001 | 0.002 |
| Glucose (mg/dl) | ||||||||||
| Menarche main effect | 0.42 | 0.08 | <.0001 | 0.32 | 0.12 | 0.01 | 0.55 | 0.11 | <.0001 | 0.19 |
| Menarche*time | 0.01 | 0.004 | 0.01 | 0.001 | 0.01 | 0.91 | 0.02 | 0.01 | 0.002 | 0.06 |
Adjusted for age and center
Statistical significance assessed at the Bonferonni corrected threshold of p<.0005.
Menarche main effect=average over the study period
Among White women only, earlier age at menarche was also associated with more adverse mean levels and changes over 25 years in HDL-C, triglycerides, blood pressure, insulin, and glucose in the minimally adjusted models (Table III). For African-American women, age at menarche was inversely associated with mean insulin levels only. There was no association between age at menarche and total cholesterol or LDL-C among either White or African-American women. Adjusting for factors other than BMI did not change estimates substantially, and so only fully adjusted Model 3 estimates are shown in Table IV. After adjusting for potential mediation by baseline BMI, each one-year earlier age at menarche was associated with a smaller increase in LDL for African-American women, but with greater overall mean triglycerides and glucose levels, and adverse changes in HDL-C, triglycerides, blood pressure, insulin, and glucose for White women only. For White compared with African-American women, associations were stronger between age at menarche and mean triglyceride levels during follow up (p-interaction race=0.0002), as well as increases over time in blood pressure (p<0.001), insulin (p=0.003), and decreases in HDL-C (p=0.0002).
Table 4.
Main effecta and per year change in cardiometabolic risk factors over 25 years according each 1-year earlier age at menarche in CARDIA, fully adjusted models
| All women N=2,583 |
African-American N=1,333 |
Whit N=1,250 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Beta | se | p-value | Beta | Se | p-value | Beta | se | p-value | p-race interaction |
| Total cholesterol (mg/dl) | ||||||||||
| Menarche main effect | −0.23 | 0.37 | 0.54 | −0.72 | 0.54 | 0.18 | 0.18 | 0.52 | 0.73 | 0.13 |
| Menarche*time | −0.02 | 0.01 | 0.12 | −0.04 | 0.02 | 0.05 | 0.01 | 0.02 | 0.54 | 0.07 |
| LDL-C (mg/dl) | ||||||||||
| Menarche main effect | −0.20 | 0.35 | 0.56 | −0.78 | 0.5 | 0.12 | 0.31 | 0.49 | 0.53 | 0.05 |
| Menarche*time | −0.02 | 0.01 | 0.06 | −0.06 | 0.02 | 0.001 | 0.01 | 0.02 | 0.48 | 0.005 |
| Menarche*time*time | ||||||||||
| HDL-C (mg/dl) | ||||||||||
| Menarche main effect | −0.27 | 0.15 | 0.08 | 0.05 | 0.21 | 0.80 | −0.57 | 0.23 | 0.01 | 0.004 |
| Menarche*time | −0.02 | 0.01 | <.0001 | 0.00 | 0.01 | 0.85 | −0.04 | 0.01 | <.0001 | 0.0002 |
| Triglycerides (mg/dl) | ||||||||||
| Menarche main effect | 1.32 | 0.45 | 0.003 | 0.13 | 0.58 | 0.82 | 2.26 | 0.68 | 0.001 | 0.0002 |
| Menarche*time | 0.14 | 0.02 | <.0001 | 0.08 | 0.03 | 0.01 | 0.20 | 0.03 | <.0001 | 0.01 |
| Systolic blood pressure (mmHg) | ||||||||||
| Menarche main effect | 0.12 | 0.12 | 0.31 | 0.06 | 0.18 | 0.76 | 0.19 | 0.15 | 0.21 | 0.34 |
| Menarche*time | 0.01 | 0.01 | 0.04 | −0.02 | 0.01 | 0.07 | 0.02 | 0.01 | 0.001 | 0.0006 |
| Diastolic blood pressure (mmHg) | ||||||||||
| Menarche main effect | 0.13 | 0.09 | 0.17 | 0.04 | 0.14 | 0.76 | 0.25 | 0.13 | 0.05 | 0.19 |
| Menarche*time | 0.02 | 0.01 | 0.0007 | −0.01 | 0.01 | 0.17 | 0.03 | 0.01 | <.0001 | <.0001 |
| Insulin (µ U/ml) | ||||||||||
| Menarche main effect | 0.10 | 0.05 | 0.06 | 0.05 | 0.09 | 0.58 | 0.16 | 0.06 | 0.007 | 0.13 |
| Menarche*time | 0.02 | 0.00 | <.0001 | 0.01 | 0.01 | 0.41 | 0.02 | 0.003 | <.0001 | 0.003 |
| Glucose (mg/dl) | ||||||||||
| Menarche main effect | 0.19 | 0.08 | 0.02 | 0.08 | 0.12 | 0.49 | 0.34 | 0.11 | 0.002 | 0.06 |
| Menarche*time | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.82 | 0.02 | 0.01 | 0.004 | 0.12 |
Adjusted for age, center, race, parental history of diabetes, education, smoking, physical activity, oral contraceptives, menopause status, alcohol intake, baseline BMI, blood pressure lowering medications, and lipid-lowering medications.
Statistical significance assessed at the Bonferonni corrected threshold of p<.0005.
Menarche main effect=average over the study period
Discussion
Our results are consistent with other studies showing associations of earlier pubertal development with adiposity and related conditions in various age groups, primarily among women of European descent [1, 2, 4–6, 10, 11, 16–22, 29, 30]. Novel aspects of our study include sufficient sample size and statistical power to report findings separately in African American and white women and the availability of serial measures of cardiometabolic risk factors captured between young adulthood and middle age.
We found that the well documented elevated level of adiposity among earlier maturing girls [4, 5, 17, 31–34] is sustained at least through mid-adulthood. Others have found a greater BMI trajectory for earlier maturing girls beginning in childhood [33] and during adolescence [13, 34]. We confirm this pattern among White women over 25 years, but not African American women. Interventions to prevent or disrupt the greater adiposity trajectory among early maturing girls might have important implications for cardiovascular health during adulthood.
We also found that White (but not African-American) women reporting late menarche between 14–17 years of age did not experience the increases in adiposity during the study that was evident for the earlier menarche groups. Later maturing White women subsequently had lower risk for T2DM, IFG, MetS, and related risk factors compared with the earlier maturing women. Others have also reported a potentially protective role of later pubertal onset (within the normal range) against obesity and cardiometabolic risk [35, 36]. The reason for this protective role is unclear, but later maturing women in CARDIA reported higher physical activity levels in high school, which may have delayed menarche [37]. Although we adjusted for physical activity level at different time points in life, residual or unmeasured confounding by other lifestyle factors are possible, and the lower weight gain and its sequelae might simply reflect long-term tracking of higher physical activity and other correlated behavioral differences among later maturing White women.
The association of earlier menarche with individual components of cardiometabolic risk after adjusting for confounding factors (in Model 2) and potential mediation by BMI (in Model 3) was specific to glucose metabolism and precursors to T2DM, and not to lipoproteins and blood pressure. Although associations did not reach statistical significance for T2DM in Models 2 or 3, possibly because of the relatively young age of the cohort and small number of diabetes cases, the current study found associations with T2DM for early vs. late menarche and per year earlier menarche that were of similar magnitude reported in other large studies (7). These patterns are consistent with other studies which report independent associations between earlier menarche and insulin-resistance factors in women [1, 4, 5, 16, 20, 31, 38, 39]. An independent association between earlier age at menarche and glucose dysregulation is biologically plausible through metabolic and hormonal changes (e.g. estrogen, progesterone, sex hormone-binding globulin) among earlier maturing girls during a window of susceptibility [5, 20, 40]. In our study, each one-year earlier menarche increased the risk for IFG by 13% and MetS by 19% among White women, independent of adiposity. Although these increases in risk are modest, they suggest that the population impact of the documented trend toward earlier maturation [7] may be great.
We found that the association between age at menarche and the cardiometabolic risk factors followed a dose-response pattern, with increasing levels of risk factors with each younger age at menarche, which agrees with the vast majority of other studies of menarche timing in relation to cardiometabolic risk factors (10, 11, 16, 17, 19). However, other studies have found a curvilinear relationship, with both early and late menarche associated with more detrimental levels of risk factors (3, 39). For example, in the ARIC study, the risk for incident diabetes among the late menarche group (15–18 years) was similar to that among the early menarche (8–11 years) group, although associations in the late menarche group did not reach statistical significance (3). One possible reason for this difference is that the women in ARIC were born during the U.S. Great Depression and as a consequence, they may have been exposed to poor nutrition during their early life. According to the ‘thrifty phenotype hypothesis’, this poor nutritional environment during fetal and infant development may have programed later chronic disease, such as T2DM, through changes in the glucose-insulin metabolism (14). Another study followed 272 suburban females starting at ages 5–22 years through age 30–46 years and found that early (<10 years) and late (>16 years) menarche were associated with MetS (39). These results might be specific to this study that was carried out in a small sample from one suburban area of Ohio, whereas the CARDIA study results may be more generalizable because it included populations from four geographically diverse communities across the United States.
We also demonstrated that associations of earlier menarche with MetS and accumulation of adiposity, higher blood pressure, and insulin were stronger among White compared with African-American women throughout adulthood both before and after adjustment for confounders (Model 2) and potential mediation by BMI (Model 3). The two-fold greater risk of incident MetS in White as compared with African American women with early menarche appeared to be driven by the stronger association of early menarche with adulthood increases in blood pressure and decreases in HDL observed in white women. At least one other study has shown that earlier menarche is more closely associated with adiposity accumulation in White compared with African-American women. The Bogalusa Heart Study [41] reported that early menarche remained an independent predictor of adulthood BMI for White but not African-American women after adjustment for childhood BMI. Furthermore, early menarche was more strongly associated with BMI among White compared with African-American adolescents, which is in agreement with our findings for change over time in adiposity for adults [31].
Despite greater overall unadjusted baseline adiposity and related cardiometabolic conditions, such as insulin and SBP, earlier menarche was not independently associated with detrimental changes over time in most risk factors for African-American women. The reason for the stronger associations for MetS and some related components among White compared with African-American women is unclear, but might relate to disparities in obesity prevalence. African-American women in all menarche categories had high adiposity overall during adulthood in our study. By year 25, the adjusted mean BMI in all three menarche categories for African- American women reached obese status (≥30 kg/m2). Data from the National Longitudinal Survey of Youth showed homogeneity in prevalence of high adiposity across menarche timing groups for African-American women starting as early as age 3 years [33]. In that study, most African-American females experienced pre-pubertal growth and maturational timing patterns similar to those experienced by White females with early menarche. Consequently, the greater degree of adiposity and cardiometabolic risk overall among African-American women may have obscured subtle associations related to menarche timing if there is some type of threshold effect.
A recent review summarizing the evidence for a relationship between earlier pubertal development and adulthood obesity and cardiometabolic risk noted that some studies suggest that earlier age at menarche simply reflects greater childhood adiposity and itself has little relevance for cardiometabolic risk, and others report that earlier maturation is an independent risk factor for adulthood obesity and other cardiometabolic risk factors [5]. Although we found near complete attenuation of results with adjustment for adiposity for T2DM, independent associations remained for other cardiometabolic risk factors. Results for these other risk factors were attenuated between 20–65% after baseline BMI adjustment, suggesting that reduction of young adult weight gain among women with early menarche may help considerably, but may not prevent all outcomes associated with early puberty.
Study limitations include that age at menarche was recalled and subject to misclassification. However, 84% of women in one longitudinal study, mean age 50, recalled their age at menarche to within one year of the actual date [42]. Women in CARDIA recalled age at menarche in early adulthood (18–30 years), and so we expect that validity was higher in our study than reported in studies of middle-age women. Nondifferential misclassification of menarche age and covariates might have biased associations, most likely toward the null. Even though we adjusted for physical activity before and during high school, it is not clear how reliably women in CARDIA recalled these activity levels. Finally, we were unable to adjust for other potential early life factors such as nutrition and body composition, which might have confounded the association between early pubertal development and later disease risk.
Strengths include the relatively large, biracial sample, which allowed the investigation of associations among African-American women that were lacking in other studies. Furthermore, the prospective design of CARDIA permitted determination of the temporality of associations and reduced several sources of bias. There were also robust, serial measures of exposures and outcomes, including objective measures for diabetes, body composition, blood pressure, and cholesterol.
Acknowledgments
We acknowledge the life-long contributions of CARDIA participants, and the study staff members, without whose commitment and enthusiasm the work of the study could never have been completed.
The Coronary Artery Risk Development in Young Adults Study is supported by the National Heart, Lung, and Blood Institute and the Intramural Research Program of the National Institute on Aging (HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, HHSN268200900041C)..J.D. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32HL007779); she currently is employed at Premier, Inc.
List of Abbreviations
- BMI
Body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CVD
Cardiovascular disease
- MetS
metabolic syndrome
- T2DM
type 2 diabetes mellitus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
References
- 1.Stockl D, Doring A, Peters A, Thorand B, Heier M, Huth C, et al. Age at menarche is associated with prediabetes and diabetes in women (aged 32–81 years) from the general population: the KORA F4 Study. Diabetologia. 2012;55:681–688. doi: 10.1007/s00125-011-2410-3. [DOI] [PubMed] [Google Scholar]
- 2.He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171:334–344. doi: 10.1093/aje/kwp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreyfus JG, Lutsey PL, Huxley R, Pankow JS, Selvin E, Fernandez-Rhodes L, et al. Age at menarche and risk of type 2 diabetes among African-American and white women in the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2012;55:2371–2380. doi: 10.1007/s00125-012-2616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heys M, Schooling CM, Jiang C, Cowling BJ, Lao X, Zhang W, et al. Age of menarche and the metabolic syndrome in China. Epidemiology. 2007;18:740–746. doi: 10.1097/EDE.0b013e3181567faf. [DOI] [PubMed] [Google Scholar]
- 5.Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.177. [DOI] [PubMed] [Google Scholar]
- 6.Elks CE, Ong KK, Scott RA, van der Schouw YT, Brand JS, Wark PA, et al. Age at menarche and type 2 diabetes risk: the EPIC-InterAct study. Diabetes Care. 2013;36:3526–3534. doi: 10.2337/dc13-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janghorbani M, Mansourian M, Hosseini E. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol. 2014;51:519–528. doi: 10.1007/s00592-014-0579-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaplowitz P. Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol. 2006;18:487–491. doi: 10.1097/01.gco.0000242949.02373.09. [DOI] [PubMed] [Google Scholar]
- 9.Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. The Journal of clinical endocrinology and metabolism. 2009;94:1527–1532. doi: 10.1210/jc.2008-2489. [DOI] [PubMed] [Google Scholar]
- 10.Lakshman R, Forouhi N, Luben R, Bingham S, Khaw K, Wareham N, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia. 2008;51:781–786. doi: 10.1007/s00125-008-0948-5. [DOI] [PubMed] [Google Scholar]
- 11.Pierce MB, Kuh D, Hardy R. The role of BMI across the life course in the relationship between age at menarche and diabetes, in a British Birth Cohort. Diabet Med. 2011 doi: 10.1111/j.1464-5491.2011.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- 14.Karapanou O, Papadimitriou A. Determinants of menarche. Reprod Biol Endocrinol. 2010;8:115. doi: 10.1186/1477-7827-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Diabetes Fact Sheet. [Accessed on January 22, 2015]; Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 16.Stockl D, Meisinger C, Peters A, Thorand B, Huth C, Heier M, et al. Age at menarche and its association with the metabolic syndrome and its components: results from the KORA F4 study. PLoS One. 2011;6:e26076. doi: 10.1371/journal.pone.0026076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kivimaki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Jarvinen L, et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns study. Am J Clin Nutr. 2008;87:1876–1882. doi: 10.1093/ajcn/87.6.1876. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, Ephross SA, Sandler DP. Menstrual patterns and risk of adult-onset diabetes mellitus. J Clin Epidemiol. 2000;53:1170–1173. doi: 10.1016/s0895-4356(00)00240-7. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. Int J Epidemiol. 2009;38:245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widen E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen AL, et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care. 2012;35:850–856. doi: 10.2337/dc11-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saquib N, Kritz-Silverstein D, Barrett-Connor E. Age at menarche, abnormal glucose tolerance and type 2 diabetes mellitus: The Rancho Bernardo Study. Climacteric. 2005;8:76–82. doi: 10.1080/13697130500062688. [DOI] [PubMed] [Google Scholar]
- 22.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. Am J Epidemiol. 1987;126:861–870. doi: 10.1093/oxfordjournals.aje.a114723. [DOI] [PubMed] [Google Scholar]
- 23.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 25.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Gross M, Steffes M, Jacobs DR, Jr, Yu X, Lewis L, Lewis CE, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51:125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 28.Yan LL, Liu K, Daviglus ML, Colangelo LA, Kiefe CI, Sidney S, et al. Education, 15-year risk factor progression, and coronary artery calcium in young adulthood and early middle age: the Coronary Artery Risk Development in Young Adults study. JAMA. 2006;295:1793–1800. doi: 10.1001/jama.295.15.1793. [DOI] [PubMed] [Google Scholar]
- 29.Sun SS, Schubert CM. Prolonged juvenile States and delay of cardiovascular and metabolic risk factors: the Fels Longitudinal study. J Pediatr. 2009;155:S7 e1–S7 e6. doi: 10.1016/j.jpeds.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller NT, Duncan BB, Barreto SM, Chor D, Bessel M, Aquino EMP, et al. Earlier age at menarche is associated with higher diabetes risk and cardiometabolic disease risk factors in Brazilian adults: Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Cardiovasc Diabetol. 2014;13:22. doi: 10.1186/1475-2840-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27:1398–1404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Zhang C, Yeung E, Ye A, Mumford SL, Wactawski-Wende J, et al. Age at menarche and metabolic markers for type 2 diabetes in premenopausal women: the BioCycle Study. The Journal of clinical endocrinology and metabolism. 2011;96:E1007–E1012. doi: 10.1210/jc.2010-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salsberry PJ, Reagan PB, Pajer K. Growth differences by age of menarche in African American and White girls. Nurs Res. 2009;58:382–390. doi: 10.1097/NNR.0b013e3181b4b921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biro FM, Lucky AW, Simbartl LA, Barton BA, Daniels SR, et al. Pubertal maturation in girls and the relationship to anthropometric changes: pathways through puberty. J Pediatr. 2003;142:643–646. doi: 10.1067/mpd.2003.244. [DOI] [PubMed] [Google Scholar]
- 35.Hartge P. Genetics of reproductive lifespan. Nat Genet. 2009;41:637–638. doi: 10.1038/ng0609-637. [DOI] [PubMed] [Google Scholar]
- 36.Mueller NT, Odegaard AO, Gross MD, Koh WP, Yuan JM, Pereira MA. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: the Singapore Chinese Health Study. Ann Epidemiol. 2012;22:717–722. doi: 10.1016/j.annepidem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavarro JE, Peterson KE, Sobol AM, Wiecha JL, Gortmaker SL. Effects of a school-based obesity-prevention intervention on menarche (United States) Cancer Causes Control. 2005;16:1245–1252. doi: 10.1007/s10552-005-0404-5. [DOI] [PubMed] [Google Scholar]
- 38.Feng Y, Hong X, Wilker E, Li Z, Zhang W, Jin D, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196:590–597. doi: 10.1016/j.atherosclerosis.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glueck CJ, Morrison JA, Wang P, Woo JG. Early and late menarche are associated with oligomenorrhea and predict metabolic syndrome 26 years later. Metabolism. 2013;62:1597–1606. doi: 10.1016/j.metabol.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen K, Aksglaede L, Munch-Andersen T, Aachmann-Andersen NJ, Petersen JH, Hilsted L, et al. Sex hormone-binding globulin levels predict insulin sensitivity, disposition index, and cardiovascular risk during puberty. Diabetes Care. 2009;32:909–914. doi: 10.2337/dc08-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 2003;3:3. doi: 10.1186/1471-2431-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol. 1991;18:155–166. doi: 10.1080/03014469100001492. [DOI] [PubMed] [Google Scholar]