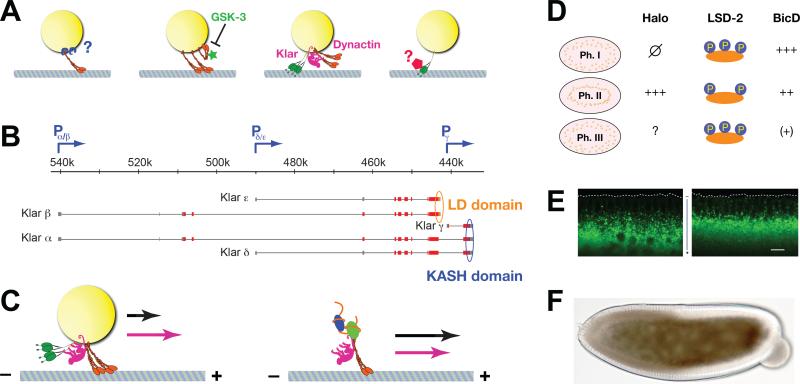
Figure 6. Regulators of lipid-droplet transport.
(A) Proposed models of force regulation during lipid-droplet transport (from left to right): 1) Motor number per droplet might be controlled by the availability of docking sites or the number of motors available for docking. The cargo adaptor for motors on droplets is not yet known [76]. 2) The activity of motors might be controlled after docking; GSK-3 has been proposed to restrict the activity of docked kinesin-1 [85]. 3) Motor coordinators may allow full force production by keeping opposite-polarity motors inactive, as proposed for dynactin and Klar [57, 84]. 4) Cytoplasmic dynein can exist in low- and high-force states [312], and these states can be controlled by transacting factors [214]. So force production by cytoplasmic dynein on lipid droplets might be regulated in vivo, an idea that has not yet been tested. (B) The complex klar locus encodes five different protein isoforms, α, β, γ, δ and ε. Promoters are indicated by blue arrows, non-coding exons by gray bars, and coding exons by red/orange/blue bars. LD domain is shown in orange, KASH domain in blue. Map modified after [266]. (C) Comparison of the effect of Klar on lipid droplet (left) and mRNA (right) transport. Arrows symbolize run lengths in the presence (pink) or absence (black) of Klar. Presence of Klar increases plus-end travel lengths for lipid droplets, but reduces them for RNP particles [57, 235]. (D) Differences in Halo, LSD-2, and BicD proteins between phases of droplet transport. Halo is absent in Phase I and expressed in Phase II; its status in Phase III is unknown, but circumstantial evidence and halo's mRNA expression pattern has led to the proposal that Halo is degraded by this time [79, 86]. LSD-2 is highly phosphorylated in Phase I and III, but less so in Phase II [87]. Droplet levels of BicD protein drop progressively from Phase I to II to III [83]. (E) Halo acts as a directionality determinant for transport. GFP-labeled lipid droplets in late Phase IIa embryos in which Halo is either expressed (left) or missing (right). The dotted line outlines the embryo surface. In the presence of Halo, net transport is plus-end directed (inward); in the absence of Halo, net transport is minus-end direction (outward). Scale bar = 10 μm. Image modified from [56]. (F) Acute effect of Halo on lipid-droplet distribution and embryo transparency. Bright-field image of a Phase IIa embryo mutant for Halo in which in-vitro generated halo mRNA was injected on the right. In the left half of the embryo, Halo activity was absent and lipid droplets are spread throughout the periphery, resulting in a broad brown “halo” around the central yolk. In the right half of the embryo, Halo activity was present, and lipid droplets accumulated around the central yolk (as a narrow dark band), leaving the periphery depleted of droplets. As a result, the peripheral cytoplasm is transparent. Image from [86].