Figure 7.
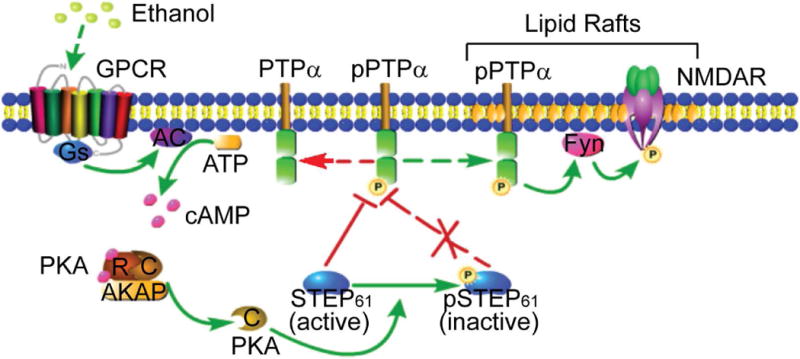
Schematic model of regulation of PTPα by STEP61 during EtOH treatment in DMS. At basal conditions, active STEP61 gates phosphorylation of PTPα and its translocation to the lipid rafts fractions where Fyn and NMDAR reside. Upon EtOH exposure, GPCR (such as D1R and A2AR) is activated, followed by activation of adenylate cyclase (AC) and production of cyclic AMP (cAMP). Binding of cAMP to the regulatory subunits (R) of PKA releases the active catalytic subunits (C) and subsequent phosphorylation of STEP61 at a regulatory site (Ser221), which is known to disrupt the interactions between STEP61 and several of its substrates. Phosphorylation of STEP61 (inactive) blocks its action on dephosphorylation of PTPα at Y789. Phosphorylated PTPα translocates to lipid rafts (maybe through binding to some adaptor proteins, with the mechanisms unclear), activates Fyn by dephosphorylating its inhibitory site (Y531) and enhances NMDAR signaling, which is implicated in EtOH-related behaviors. The mechanisms underlying the region specificity (i.e. DMS versus DLS and NAc) remain unclear. We propose that distinct PKA regulatory and catalytic subunits and region-specific or compartment-specific distribution of AKAPs may be involved. GPCR, G-protein coupled receptor; D1R, dopamine D1 receptor; A2AR, adenosine A2A receptor; NMDAR, N-methyl-D-aspartate receptor; R, regulatory subunit; C, catalytic subunit; AKAP, A-kinase anchor protein; DMS, dorsomedial striatum; DLS, dorsolateral striatum; NAc, nucleus accumbens. This figure was created with the aid of the Pathway Builder Tool 2.0 (www.proteinlounge.com).
