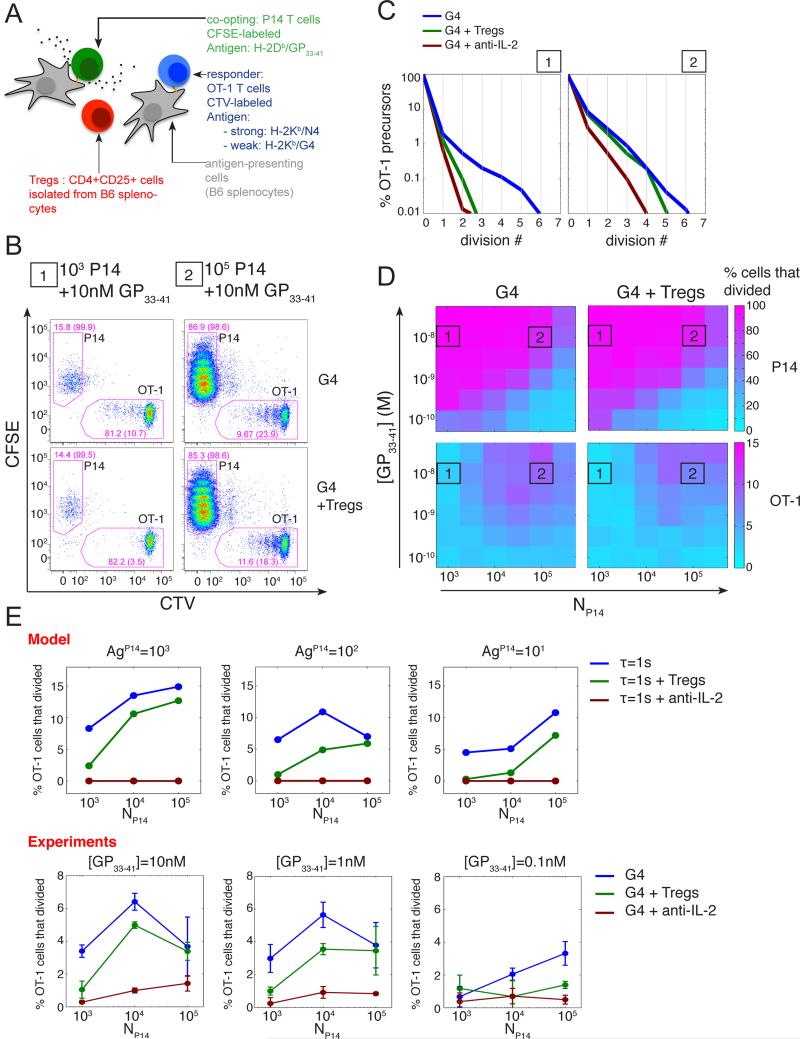
Fig. 5. Tregs can modulate IL-2 mediated inter-clonal co-optation of weak T cell clones.
(A) CD4+CD25+ Tregs cells were added to the in vitro co-culture system introduced in Fig. 1A.
(B) Representative dot plot of CFSE and CTV MFI for live CD8+ cells after 3 days of co-culture in absence or presence of Tregs cells (isolated as CD4+CD25+ from B6 splenocytes). OT-1 cells were stimulated by APCs pulsed with the weak agonist G4 (1uM) in the presence of different quantities (condition 1, 103; condition 2, 105) of P14 cells stimulated by APCs pulsed with their cognate antigen GP33-41 (10nM). Drawn gates identify P14 and OT-1 populations within live CD8+ cells and their respective frequencies are indicated. For each population, the percentage of cells that divided at least once is also indicated in parentheses.
(C) Distributions of the number of cell divisions per OT-1 precursor cells after 3 days in the in vitro co-culture system for the co-culture conditions 1 and 2 presented in (B) in the presence or absence of Tregs or IL-2 blockade. At low density of ‘co-opting’ P14 cells, Tregs cells suppress the co-optation of weakly stimulated OT-1 cells as efficiently as a direct block of IL-2 and this effect vanishes as the density of ‘co-opting’ cells increases.
(D) Representation of the percentage of P14 and OT-1 precursors cells that divided, in the presence or absence of Tregs cells, as a function of the density of ‘co-opting’ P14 cells and the concentration of their cognate antigen GP33-41 used to pulse the corresponding APCs. The conditions 1 and 2 referenced in (B) and (C) are indicated.
(E) The hybrid model correctly predicts the dependency of the suppression of the inter-clonal co-optation by Tregs as a function of the co-opting cell density. Representation of the percentage of OT-1 precursor cells that divided as a function of the density of co-opting P14 cells for different quantities of their cognate antigen GP33-41 and in the presence or absence of Tregs and IL-2 blockade. Model: OT-1, P14 and Tregs cells were simulated simultaneously using replicates of the model presented in Fig.3. OT-1 cells were stimulated by a weak agonist (τ=1s). In the case of P14 cells, the cognate antigen was taken as a strong agonist with parameter τ=10s. For Tregs, no specific antigen was introduced but we considered a high basal transcription rate of the IL-2Rα gene to generate the high abundance (set here to 103 copies per cell in the basal state) of IL-2Rα characteristic of this population. Simulation results shown were obtained with 104 Tregs per condition. For each condition, the percentage of cells that divided was estimated from 103 independent simulations. Experiments: cells were co-cultured for 3 days as in (B-D). Error bars represent mean +/− SD for two replicates; the results are representative of n≥3 independent experiments.