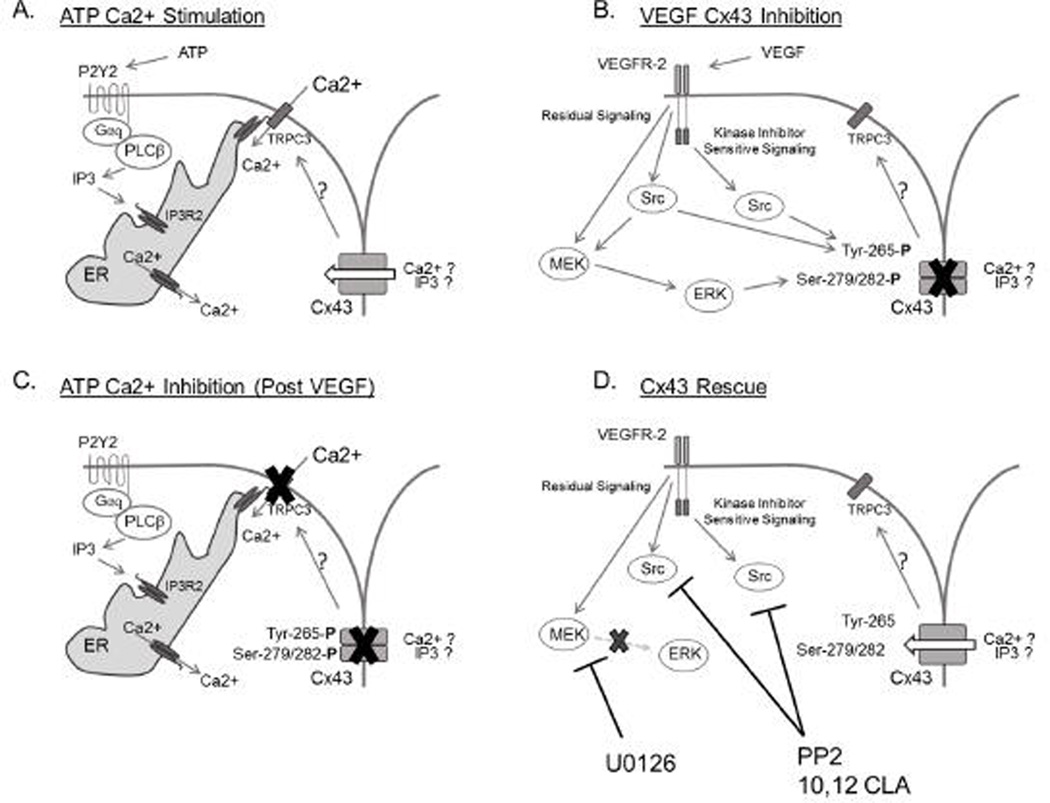
Figure 8. Proposed mechanism for ATP stimulation, VEGF inhibition, and subsequent recovery of sustained Ca2+ responses in P-UAEC.
Panel A depicts a typical ATP-stimulated Ca2+ response, as described previously (Boeldt, et al. 2011; Yi, et al. 2010a). Most notably, the initial Ca2+ response consists of IP3-regulated endoplasmic reticulum Ca2+ release into the cytosol. When the endoplasmic reticulum Ca2+ levels are sufficiently low, IP3R2 interacts with TRPC3 at the cell surface, opening it and allowing extracellular Ca2+ influx. This process is potentiated by Cx43 function through an as yet unknown mechanism, though likely candidates include IP3 or Ca3+ gating in close proximity to TRPC3. Panel B is now derived from previous studies (Grummer et al. 2009) and those described here concerning VEGF-stimulated signaling responsible for acute inhibitory phosphorylation of Cx43 at Ser-279/282 and Tyr-265. Of note, Src family kinase signaling originates from both classic kinase domain signaling and ‘residual’ VEGFR-2 signaling. Our data in particular suggests MEK/ERK signaling is derived exclusively from ‘residual’ VEGFR-2 signaling, through direct signaling or through secondary events downstream of a Src kinase. Panel C shows the consequence of VEGF-stimulated Cx43 phosphorylation on subsequent ATP-stimulated Ca2+ responses. The phosphorylation of Cx43 at Ser-279/282 and Tyr-265 render Cx43 non-functional and thus the transmission of signals necessary for sustained phase Ca2+ responses are lost. The result is a normal initial Ca2+ response to ATP stimulation through release from the ER, but a significant reduction in the sustained-phase Ca2+ bursts that follow. In panel D, mechanisms of action for pharmacological inhibitors of MEK/ERK and Src family kinases are shown. Blockage of MEK/ERK and Src kinases prevents inhibitory phosphorylation of Cx43 at Ser-279/282 and Tyr-265 and so prevents the inhibitory activity of VEGF pretreatment and restores sustained-phase Ca2+ bursts in response to ATP.