Abstract
Novel cell surface-reactive monoclonal antibodies generated against extrahepatic biliary cells were developed for the isolation and characterization of different cell subsets from normal adult human gallbladder. Eleven antigenically distinct gallbladder subpopulations were isolated by fluorescence-activated cell sorting. They were classified into epithelial, mesenchymal, and pancreatobiliary (PDX1+SOX9+) subsets based on gene expression profiling. These antigenically distinct human gallbladder cell subsets could potentially also reflect different functional properties in regards to bile physiology, cell renewal and plasticity. Three of the novel monoclonal antibodies differentially labeled archival sections of primary carcinoma of human gallbladder relative to normal tissue. The novel monoclonal antibodies described herein enable the identification and characterization of antigenically diverse cell subsets within adult human gallbladder and are putative tumor biomarkers.
Keywords: Gallbladder, Cystic duct, Extrahepatic Biliary, Monoclonal antibodies, Gallbladder carcinoma
INTRODUCTION
The human gallbladder mainly functions to concentrate and store bile, and like anatomically close-by organs—the liver and the pancreas——is derived from the foregut endoderm during embryonic development [1]. The extrahepatic biliary tree (comprised of extrahepatic bile ducts, common duct, cystic duct and gallbladder) shares a common progenitor with the ventral pancreas during embryonic development [1]. In the adult, putative facultative stem/progenitor cells are postulated to exist in injured liver [2–6], pancreas [7], and the extrahepatic biliary system [8–12].
The development of cell surface markers that label different cell subsets in the liver and pancreas has facilitated greater understanding of these cells and their roles within their respective organ systems [2, 3, 13]. In contrast, no cell surface markers have been developed specifically for normal adult human gallbladder or the extrahepatic biliary tree to date. The availability of antibodies that preferentially mark cell populations within the extrahepatic biliary system would allow the identification and classification of these cells in relation to bile physiology, gallstone formation, cell plasticity and renewal, metaplasia, and tumor formation.
Here, novel monoclonal antibodies were developed to detect cells originating from human extrahepatic biliary tissues (EHBT) such as gallbladder and cystic duct. These monoclonal antibodies allowed the visualization and isolation of antigenically diverse subpopulations from adult human gallbladder. Gene expression analyses of these extrahepatic biliary cell (EHBC) subsets further indicated distinct epithelial, mesenchymal, or pancreatobiliary traits. Finally, survey of several primary carcinomas of human gallbladder showed differential immunolabeling with respect to normal gallbladder.
MATERIALS AND METHODS
Gallbladder and cystic duct specimens
Normal human gallbladder and cystic duct specimens (collectively referred to as extrahepatic biliary tissues or EHBT) were sourced from the Departments of Surgery and/or Surgical Pathology at the Oregon Health & Science University (OHSU). The pathologist on duty selected and excised grossly normal EHBT fragments (1 cm2 to 3 cm2). These were kept on ice in DMEM/F12 supplemented with 100 units/mL Penicillin and 100 μg/mL Streptomycin (Mediatech, Manassas, VA, USA). Tissue samples were anonymized as outlined in the IRB study exemption approved by the OHSU Institutional Review Board. Single cell suspensions of gallbladder and cystic duct (collectively called extrahepatic biliary cells or EHBC) were prepared by digestion of EHBT in 5 mg/mL collagenase II (Gibco, Grand Island, NY, USA) in 1X HBSS (HyClone Laboratories, South Logan, UT, USA), 20 μg/mL DNaseI (Roche, Laval, QC, Canada) and 1% v/v HEPES (Mediatech, Manassas, VA, USA) for a total of 2 hour incubation in a 37°C water bath. EHBT tissue chunks were subsequently strained through a 100-micron nylon mesh (BD Falcon, Bedford, MA, USA) and cells washed and treated with ACK lysis solution (0.15M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4, filter-sterilized) for 2 minutes at room temperature to remove red blood cells. Finally, EHBC were washed and strained using a 40-micron nylon mesh (BD Falcon, Bedford, MA, USA) and resuspended in DMEM/F12 supplemented with 0.5% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and kept on ice. To archive tissues, EHBT samples were cut into less than 1 cm2 fragments and embedded in Tissue-tek cryomatrix (Sakura, Tokyo, Japan) and stored at −81°C. Formalin-fixed paraffin-embedded (FFPE) tumor sections from anonymized primary human adenocarcinoma of the gallbladder were obtained from the OHSU Knight BioLibrary.
Antibody production
Animal husbandry and immunizations adhered to OHSU Institutional Animal Care and Use Committee (IACUC) under approved protocol A982. Female Balb/c mice (10 weeks old) were immunized with 3 doses by intraperitoneal injection of freshly isolated viable EHBC (5×105–3×106 cells per dose) given 3 weeks apart. Mice were sacrificed four days after the last dose and splenocytes were harvested and fused with SP2/0 Ag14myeloma cells. Hybridoma clones were grown in methylcellulose-containing HAT medium (Stem Cell Technologies, Vancouver, BC, Canada) [13]. A total of 427 isolated hybridoma clones were generated, picked, and transferred to 96-well plates. Neat supernatants were collected for screening by indirect immunofluorescence on acetone-fixed frozen sections of human EHBT. Clones of particular interest (Table 1) were cryopreserved and expanded in culture in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% v/v fetal bovine serum. Antibody isotypes were determined using a direct horseradish peroxidase-based mouse immunoglobulin isotyping ELISA kit (BD Biosciences, San Diego, CA, USA).
Table 1.
Monoclonal antibodies reactive to antigens in human extrahepatic biliary cells (EHBCs)
| Antibody | Immunofluorescence staining patterns in gallbladder and cystic duct | Antibody isotype (Mouse) | Frequency of positive cells
|
|
|---|---|---|---|---|
| Gallbladder | Cystic duct | |||
| GB1* | Subset of mucosal epithelium | IgG | 2% | 1% |
| GB2* | All Mucosal epithelium with apical bias | IgG | 8% | 3% |
| GB3* | Subset in the muscularis layer; subset of peribiliary glands | IgM | 6% | 6% |
| GB4* | All Mucosal epithelium + peribiliary glands and surrounding connective tissues | IgG | 5% | 2% |
| GB5* | Mucosal epithelium with basal bias | IgG | 5% | 2% |
| GB6* | All Mucosal epithelium + subset of lamina propria + peribiliary glands and surrounding connective tissues | IgM | 80% | 80% |
| GB7* | Mucosal epithelium with subcellular localization | IgM | 1% | <1% |
| GB8* | Mucosal epithelium with subcellular localization | IgM | 2% | 1% |
| GB9 | Subset of mucosal epithelium | IgM | 6% | 2% |
| GB10 | Subset of mucosal epithelium with apical bias + peribiliary glands | IgG | 3% | 2% |
| GB11 | Mucosal epithelium | IgM | 7% | 2% |
| GB12 | Mucosal epithelium with apical bias | IgG | 10% | 3% |
| GB13 | Mucosal epithelium | IgG | 9% | 3% |
| GB14 | Secreted/Luminal | N.D. | 0 | 0 |
| GB15 | Circumference of large blood vessel walls | IgM | <1% | <1% |
| GB16 | Mucosal epithelium, peribiliary glands | IgG | 4% | 2% |
| GB17 | Subset of mucosal epithelium with apical bias | IgM | 2% | 1% |
| GB18 | Mucosal epithelium with apical bias; peribiliary glands | IgG | 7% | 2% |
| GB19 | Subset of mucosal epithelium with apical bias | N.D. | 0 | 0 |
| GB20 | Mucosal epithelium and subsets in the muscularis layer | IgG | 8% | 4% |
Antibodies marked with asterisks (*) were selected for dual labeling fluorescence-activated cell sorting to subdivide adult human EHBCs. GB=human gallbladder. N.D. not determined
Immunofluorescence
Five-micron thick cryosections of adult human EHBT were prepared using a Reichert 2800 Frigocut cryostat (Reichert Scientific Instruments, Buffalo, NY, USA). Cryosections were fixed in acetone for 5 minutes at −20°C, allowed to dry at room temperature, and stored at −81°C for up to three months. One hundred microliter volume of hybridoma supernatants were utilized to label EHBT sections, washed with PBS, and followed by staining with 0.5 μg/μl of Cy3- or AlexaFluor 488-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA) in PBS supplemented with 5% (v/v) fetal bovine serum (HyClone Laboratories, South Logan, UT, USA) and 5% (v/v) Rat Serum (AbD Serotec, Raleigh, NC, USA). Slides were washed with 1X PBS prior to mounting with a solution containing 10% glycerol and 4% N-propyl gallate with 0.001% (v/v) Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA). Slides were evaluated using a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Jenna, Germany).
For pancytokeratin (PanCK) staining, a rabbit polyclonal antibody for wide-spectrum cytokeratin (Abcam, Cambridge, MA, USA) was used in combination with GB1, GB5, and HPd3 following the standard use of hybridoma supernatants in indirect immunofluorescence of acetone-fixed EHBT stated above.
For FFPE-tumor sections, slides were heated to 60°C for 15 min. Sections were deparaffinized in xylene at room temperature for 15 min and rehydrated by soaking for 4 minutes at room temperature in various concentrations of ethanol (100%, 95%, 70%, and 50%), rinsed in deionized water and stored in 1X PBS (Fisher Scientific, Fish Lawn, NJ, USA). For antigen retrieval, sections were immersed in citrate buffer (10 mM citrate, pH 6.2, 2 mM EDTA, 0.05% Tween-20) using glass staining dish and heated in a microwave oven for 3 consecutive 5-min cycles at power levels 5, 5, and 4. The dish was allowed to cool down to room temperature and tissue sections were rinsed twice in 1X PBS [14]. Sections were blocked with 5% v/v normal goat serum (Jackson ImmunoResearch, West Grove, PA, USA) in 1X PBS for 2 hours at room temperature, and rinsed twice with PBS prior to immunohistochemical labeling as above.
Flow cytometry
Dissociated EHBC were incubated in hybridoma supernatant for 30 min at 4°C and washed with cold DMEM prior to labeling with anti-mouse secondary antibodies, namely: PE-conjugated anti-mouse IgM and DyLight488-conjugated anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA). Propidium iodide (10 μg/mL) (Molecular Probes, Eugene, OR, USA) was included to the cell suspensions to mark dead cells. Cells were analyzed with a FACSCalibur or sorted by Influx-GS (BD Biosciences, San Jose, CA, USA) at 15 psi using a 100-μm nozzle. The software FlowJo (Treestar, Ashland, OR, USA) was utilized to analyze flow cytometric data.
RT-qPCR
FACS-sorted cells were collected into Trizol LS (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted by following the manufacturer’s recommended protocol. First strand cDNA was synthesized utilizing random oligonucleotides and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) as directed by the manufacturer. SYBR-based quantitative PCR was performed by utilizing either iQTM 5 or CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) to measure gene expression. The qPCR reactions were comprised of Platinum® Taq polymerase, 2.5 mM MgCl2, 10 μM DNA primers, 10 mM dNTPs, and 0.5X SYBR green (Invitrogen, Carlsbad, CA, USA). Thermocycling reactions were run as follows: 45 cycles of 15 s at 95°C, 20 s at 68°C, and 25 s at 72°C. The following are the primers used: LMNA (5′, agatgcgggcaaggatgcag, 3′, cctcctcgccctccaagagc), ACTB (5′, cacccgccgccagctcac, 3′, atcacgccctggtgcctggg), RPL32 (5′, tctccttctcggcatcatggccg, 3′, tgggtttccgccagttacgct), EPCAM (5′, ttgccgcagctcaggaagaa, 3′, tttggcagccagctttgagc), MUC5B (5′, ctgggagaatgcagggcaca, 3′, ggctcaggctggggaagaca), PDX1 (5′, tggaggagcccaaccgcgtccagc, 3′, gcgccgcctgcccactggcctt), CCKAR (5′, gcaggcaaggatggatgtgg, 3′, ccagcacgctgagcaggaat), MYH11 (5′, gggcggagctcaatgacaaa, 3′, aagcagctcctgggtgtcctg), KRT19 (5′, cctcccgcgactacagccacta, 3′, ccacttggcccctcagcgta), VIM (5′, agctcaagggccaaggcaagtc, 3′, tctcctcctgcaatttctcccg), SOX9 (5′, gcggcccttcgtggaggaggcgga, 3′, tgggattgccccgagtgctcgccgg). Gene expression values were calculated as the difference between baseline-corrected, curve-fitted threshold cycles (Cq) of the genes of interest subtracted by the mean Cq of reference genes (LMNA, RPL32, ACTB).
RESULTS
Antibody Screening of human gallbladder and cystic duct
A total of 427 hybridomas were produced after serial immunization of Balb/c mice with dispersed cells from human extrahepatic biliary tissues (EHBT). Antibody-containing supernatants from each hybridoma clone were then screened for reactivity by indirect immunofluorescence in acetone-fixed EHBT sections (adult human gallbladder and cystic duct). The frequency of clones with extrahepatic biliary tissue labeling was 36.8% (157 out of 427). The majority of the 157 positive mAbs stained cell subsets (147/157), while the remainder labeled the entire gallbladder or cystic duct tissue sections (10/157).
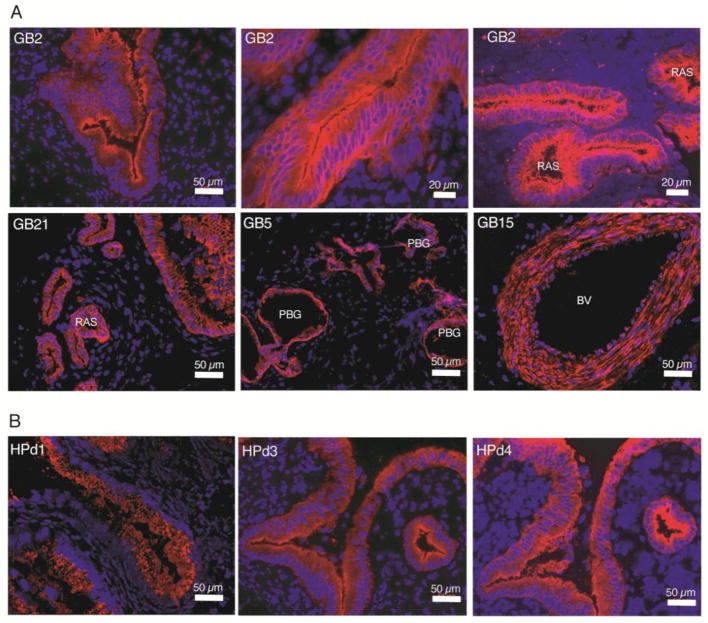
The observed immunostaining patterns were consistent with areas of (a) mucosal epithelium characterized by apical/luminal staining and/or basolateral staining, (b) Rokitansky-Aschoff sinuses (RAS) [15], (c) generalized or patchy staining encompassing the mucosa, lamina propria, muscularis and the adventitial or subserosal layers, (d) submucosal structures in the cystic duct called peribiliary glands (PBGs), and (e) blood vessel walls (BV) (Figure 1A, Table 1 and Suppl. Fig. 1–2).
Figure 1. Immunofluorescence labeling patterns in adult human gallbladder.
A) The mAb GB2 labeled mucosal epithelia (top left and top middle panels) with characteristic outpouching (also known as Rokitansky-Aschoff sinuses (RAS)) (top right) extending into the muscularis layer. Also, GB21 selectively labeled mucosal epithelium (bottom left), but without the apical bias of GB2. GB5 mAb marked peribiliary glands (PBGs) (bottom middle). GB15 labeling of a blood vessel (BV) wall (bottom right). B) Three pancreatic ductal surface antibodies labeled gallbladder mucosal epithelium.
Flow cytometric analyses in dispersed gallbladder and cystic duct cells
Seventy-eight mAb clones were selected for further analyses by flow cytometry based on staining intensity and immunolabeling of discrete groups of cells found in the mucosal, muscular, and adventitial/serosal layers (Figure 1A and Suppl. Fig. 1–2). Further cross-referencing the immunolabeling patterns and intensities of the 78 mAbs yielded 20 distinctive mAbs, which were analyzed in single cell suspensions of live extrahepatic biliary cells (EHBCs) from both gallbladder and cystic duct to determine cell surface reactivity. Seventeen of the 20 mAbs had surface reactivity in at least 1% of cells while one mAb labeled less than 1% of total EHBCs. The remaining two mAbs failed to label live dispersed EHBCs (Table 1).
Several of the antibodies raised (GB1, 2, 5, 7, 8, 9, 11, 12, 13, and 17) were useful for FACS-mediated isolation of purely epithelial cells derived from the gallbladder and cystic duct (Table 1). Importantly, some antibodies specifically labeled only subpopulations of the mucosal epithelium (GB1, 9, and 17), demonstrating that the epithelial layer of the gallbladder mucosa is comprised of antigenically heterogeneous cell types, not obvious with hematoxylin and eosin staining or even pancytokeratin immunolabeling (Figure 3).
Comparative immunofluorescence of EHBT and human pancreas
Given the anatomic and developmental proximity of the EHBT to the pancreas, we tested surface antibodies generated against human cytokeratin-19+ pancreatic ductal cells (HPd1 and HPd3) [13, 16] were also tested on acetone-fixed sections of EHBTs, along with another pancreatic duct marker HPd4 and our new antibodies. All three pancreatic duct mAbs also labeled the epithelial cells of the gallbladder mucosa (Figure 1B).
Twenty-nine of the gallbladder mAbs were further tested in acetone-fixed human pancreatic tissue sections with 27 mAbs labeling extensive areas of the pancreas (e.g. ducts, exocrine cells, blood vessels, and Islets of Langerhans) (Suppl. Fig. 3). The remaining two mAbs (GB26 and 27) labeled sparse areas (small subsets of cells) in the human pancreas. These data infer that majority of these mAbs generated against EHBT cross-react with pancreatic cells suggesting shared antigens with gallbladder and cystic duct.
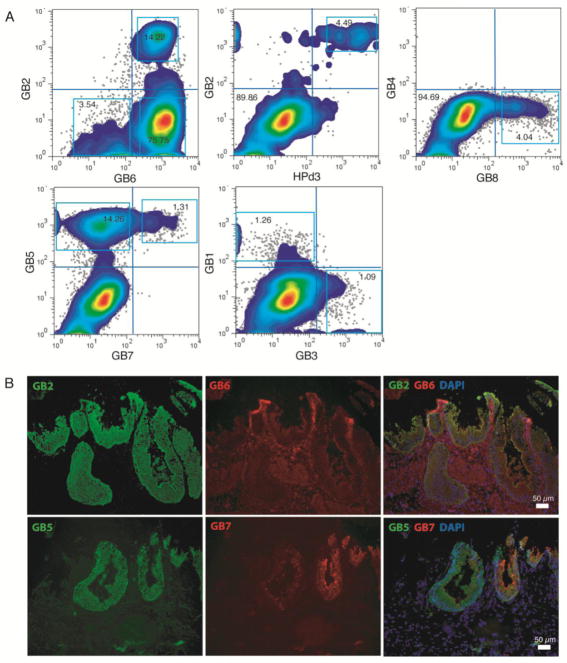
FACS isolation of gallbladder subpopulations
Next, the list of twenty mAbs was further narrowed to eight GB mAbs for fluorescence-activated cell sorting (FACS) in combination with the pancreatic ductal mAb HPd3 to potentially subdivide EHBCs into discrete subpopulations (Figure 2A). For dual labeling, combined IgG and IgM primary antibodies were distinguished using isotype-specific secondary antibodies (Table 1). This method identified eleven clearly distinct subpopulations in live EHBCs (Figure 2A and Table 2). Dual immunofluorescence in acetone-fixed sections of human gallbladder (Figure 2B) was able to visualize these same populations and their in situ frequency was similar to their abundance as measured by FACS in dispersed cell suspensions (Figure 2A).
Figure 2. Isolation of subpopulations from adult human gallbladder.
A) Flow cytometric dotplots of human EHBC subsets subdivided by GB2 and GB6 (top left), GB2 and HPd3 (top middle), GB4 and GB8 (top right), GB5 and GB7 (bottom left), and GB1 and GB3 (bottom middle). B) Dual-immunofluorescence staining of acetone-fixed EHBT. Right panels depict merged images.
Table 2.
Immunofluorescence labeling frequencies and gene expression analysis of FACS-sorted extrahepatic biliary cells
| Subpopulations | Frequency of sorted cells* | Relative mRNA expression§ | Gene expression summary# | ||||
|---|---|---|---|---|---|---|---|
| EPCAM | KRT19 | SOX9 | PDX | VIM§ | |||
| VIM | VIM | VIM | EPCAM | ||||
| GB6+GB2− | 75.8 % | 0 | 8.8e-6 | 9.8e-3 | ∞ | 1.08 | VIMhi, MYH11hi (mesenchymal) |
| GB6+GB2+ | 14.2 % | 6.14 | 9.03 | 2.51 | 2.56 | 0.11 | EPCAM+, SOX9hi, KRT19hi, MUC5B+, PDX1+ (mixed epithelial) |
| GB6−GB2− | 3.5 % | 0.065 | 0.21 | 0.067 | 0 | 1.35 | VIMhi, MYH11+ (mesenchymal) |
| HPd3−GB2− | 89.9 % | 1.5e-4 | 7.7e-4 | 3.7e-3 | 0.34 | 1.25 | VIMhi (mesenchymal) |
| HPd3+GB2+ | 4.5 % | 51.2 | 86.1 | 20.5 | 0.016 | 0.013 | EPCAM+, SOX9hi, KRT19hi, MUC5B+, PDX1+ (mixed epithelial) |
| GB1+GB3− | 1.3 % | 24.6 | 17.7 | 13.0 | 0.064 | 0.022 | EPCAM+, SOX9hi, KRT19+, MUC5B+, PDX1hi (mixed epithelial) |
| GB1−GB3+ | 1.1 % | 1.07 | 0 | 8.1e-3 | 0 | 3.43 | EPCAMhi, VIMhi, MUC5Bhi (mixed epithelial-mesenchymal) |
| GB8+GB4− | 4.0 % | 11.6 | 13.2 | 6.18 | 0.061 | 0.077 | PDX1hi, SOX9hi, CCKARhi, (pancreatic-biliary) |
| GB8−GB4− | 94.7 % | 0.057 | 0.074 | 0.024 | 0.024 | 1.30 | VIMhi, MYH11hi (mesenchymal) |
| GB5+GB7+ | 1.3 % | ∞ | ∞ | ∞ | 0.30 | 0 | PDX1hi, SOX9hi, MUC5Bhi (pancreatic-biliary) |
| GB5+GB7− | 14.3 % | 23.3 | 25.0 | 5.82 | 8.8e-3 | 0.034 | EPCAM+, KRT19+, SOX9+ (epithelial) |
| Unsorted EHBC | n.a.⌘ | 0.14 | 0.89 | 0.058 | 9.1e-3 | 1.77 | KRT19hi, MYH11hi, VIMhi, CCKARhi (mixed epithelial-mesenchymal) |
| EHBT | n.a⌘ | 0.59 | 3.25 | 4.1e-3 | 7.4e-4 | 3.58 | EPCAMhi, KRT19hi, MYH11hi, VIMhi, CCKARhi (mixed epithelial-mesenchymal) |
As percentage of live extrahepatic biliary cells (EHBCs); Live cells were sorted as propidium iodide (PI) negative cells.
Relative mRNA expression was calculated based on qPCR ΔCq values, which are the differences between the Cq of the gene of interest and the Cq of housekeeping genes LMNA, RPL32, and ACTB.
Mesenchymal characteristic was designated if the ratio of epithelial/ductal markers (EPCAM, KRT19, SOX9) to Vimentin was less than one, otherwise, a designation of predominantly epithelial was given. In addition, a pancreatic or gallbladder designation was given if relative expression of “pancreatic” (PDX1) or “gallbladder” genes (MUC5B, CCKAR) was greater than that of unsorted EHBCs.
n.a.: not applicable
Gene Expression Analysis
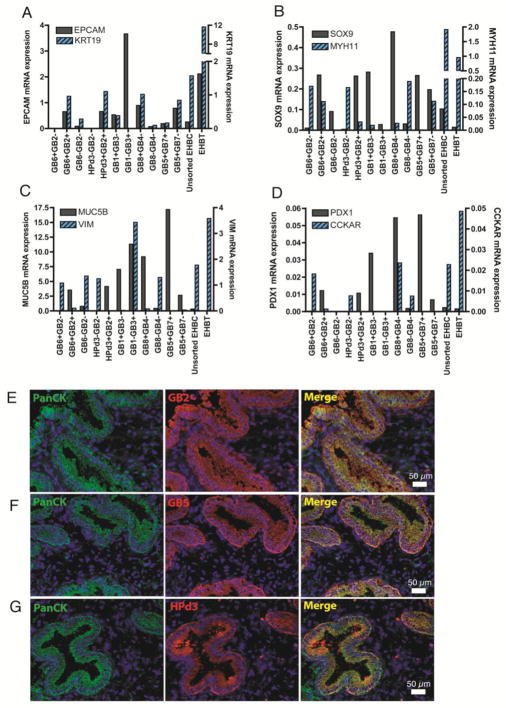
The eleven antigenically distinct EHBC subsets isolated by FACS had distinct gene expression patterns by RT-qPCR and could be characterized as predominantly epithelial (epithelial (EPCAM+, KRT19+)), ductal (SOX9+), mesenchymal (VIM+, MYH11+), gallbladder (MUC5B+, CCKAR+), or pancreatobiliary (PDX1+SOX9+) (Figure 3A–D and Table 2).
Figure 3. Gene expression characteristics of human EHBC subsets via RT-qPCR.
Bar graphs depict the relative mRNA expression of human EHBC cell subsets, as measured by the following genes of interests: EPCAM and KRT19 (A), SOX9 and MYH11 (B), MUC5B and VIM (C), PDX1 and CCKAR (D). Gene expression levels were normalized to reference genes LMNA, RPL32, and ACTB. Immunofluoresecence co-labeling of Pancytokeratin (PanCK) with GB2 (E), GB5 (F), and HPd3 (G) in acetone-fixed sections of adult human gallbladder.
Mixed epithelial subsets
Six different EHBC subsets (GB6+GB2+, HPd3+GB2+, GB1+GB3−, GB8+GB4−, GB5+GB7+, and GB5+GB7−) were similarly enriched for MUC5B, SOX9, and PDX1 mRNA relative to unsorted EHBC and EHBT (Figure 3B–D). Immunofluorescence of EHBT acetone-fixed sections showed co-labeling of pancytokeratin with GB2, GB5, and HPd3 (Figure 3E–G), but not with GB7. Furthermore, VIM expression was very low to absent in these EHBC subtypes (Figure 3C). Together, these data demonstrate that these six cell populations represent a subset of epithelial cells with low expression of EPCAM and KRT19 but high levels of SOX9, MUC5B and PDX1.
PDX1+SOX9+ subsets
Notably, the GB8+GB4− and GB5+GB7+ subsets differed from other fractions in that they had very high PDX1 mRNA levels measured at 33.4% and 34.6% compared to human pancreatic islet cells. Moreover, the PDX1 mRNA expression levels in these populations were 23-fold and 24-fold enriched compared to unsorted EHBCs, respectively. Similarly, another subset (GB1+GB3−) also had 14.5-fold enrichment of PDX1 mRNA with respect to unsorted EHBCs (Figure 3D). Furthermore, these same three PDX1+ subsets had 2.5-fold to 4.6-fold higher expression of SOX9 compared to unsorted EHBCs (Figure 3B).
Mixed epithelial-mesenchymal subset
Interestingly, the subpopulation GB1−GB3+ (which we designated as mixed epithelial-mesenchymal) (Table 2), had a relatively high EPCAM, MUC5B, and VIM mRNA content but absent expression of KRT19, SOX9, and PDX1 (Figure 3A–D). This is consistent with the observation that GB3 marks a subset in the peribiliary glands and the muscularis layer (Table 1).
Human Gallbladder Adenocarcinoma
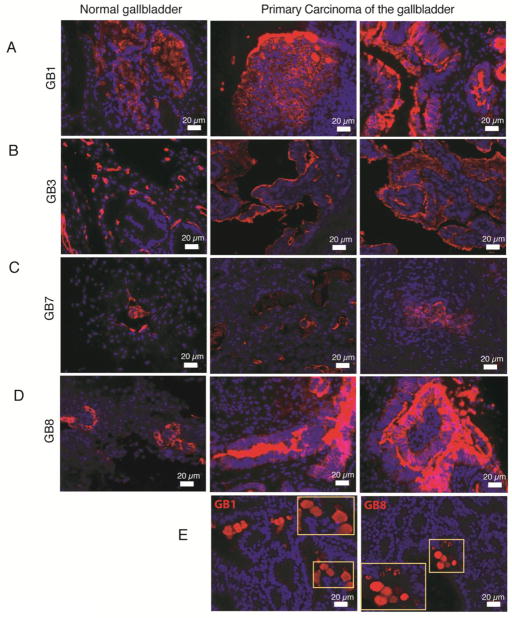
In addition to the identification of antigenically heterogeneous epithelial subpopulations in EHBT, we were interested in the cellular subset distribution of tumors. Our eleven novel surface-reactive antibodies were tested in formalin-fixed, paraffin-embedded (PPFE) sections of 5 primary adenocarcinoma arising in the human gallbladder (GBCA) (Suppl. Table 1). Three different types of antigen-retrieval procedures were performed in FFPE sections of both primary tumors and normal tissues from human gallbladder, but only 5 mAbs (GB1, GB3, GB7, GB8, and HPd3) successfully worked to label FFPE sections (Figure 4).
Figure 4. Immunofluorescence labeling of formalin-fixed paraffin-embedded (FFPE) human gallbladder.
PPFE sections of both normal (left panels) and primary carcinoma (middle and right panels) were amenable to immunofluorescence with GB1 (A), GB3 (B), GB7 (C), and GB8 (D). Tumor samples labeled with GB1 and GB8 were found to have distinctive circular extracellular aggregates (E).
GB3 staining in tumors
The antigen stained by GB3 was evenly distributed in a patchy fashion throughout the different layers of normal gallbladder, particularly areas in proximity to the basement membrane of mucosal epithelium, small blood vessel walls in the muscularis, and areas in between (Figure 4B, left panel). However, this regular labeling pattern was lost in the four (out of five) primary adenocarcinomas of human gallbladder (GBCA) tested. Instead, GB3 labeling was restricted to the luminal areas of mucosal epithelium proliferation and some areas near blood vessels (Figure 4B, middle and right panels). This differential antigen localization (basal to luminal) may be a potential marker of malignancy or tumor-associated antigen in the gallbladder.
GB1 and GB8 staining in tumors
Similarly, two other antibodies (GB1 and GB8) had marked luminal-staining bias in tumor sections (Figure 4A, D, middle and right panels), which was less evident in normal gallbladder tissues (Figure 4A, D, left panels). In one invasive GBCA sample, distinctive extracellular circular bodies were found in the luminal space and were stained by both GB1 and GB8 antibodies (Figure 4E).
GB7 Antibody
GB7 labeling of FFPE sections of both normal and tumor samples was not drastically different in the small sample size of primary gallbladder tumors tested (Figure 4C).
DISCUSSION
We have generated novel monoclonal antibodies from human EHBT that are useful for the visualization and isolation of antigenically diverse subpopulations from adult human gallbladder and cystic duct. The patient-derived material for immunization was prepared to enrich for epithelial cells, so it was not surprising to see that majority of the antibodies labeled mucosal epithelia in EHBTs. The immunostaining patterns in both gallbladder and cystic duct indicate antigenic heterogeneity in the mucosal epithelium, which may further signify functionally distinct subsets. Moreover, submucosal structures in the cystic duct called peribiliary glands or PBGs were easily identifiable by some of these mAbs. PBGs had been shown to harbor biliary progenitor cells that are capable of differentiating into hepatocyte-like, cholangiocyte-like, or pancreatic islet-like cells [9, 11]. FACS-enrichment of cells comprising PBGs is feasible with our surface-reactive mAbs.
Our new mAbs were tested primarily in extrahepatic biliary cells from the gallbladder and cystic duct because these structures are some of the most accessible for research (anatomically and specimen availability) within the hepatobiliary tree. We have no direct evidence as to the specificity of these mAbs to only the extrahepatic biliary cells versus intrahepatic biliary cells. Given the anatomic continuity of the EHBT to the intrahepatic biliary tree (IHBT), we predict that some of these mAbs may react with the IHBT. Several groups had shown that PBGs in the EHBT are marked by CK7, CK19, NCAM, CD133, EPCAM, SOX9, and SOX17, which are also some of the major markers of IHBT [9, 17]. On the other hand, another study showed that human intrahepatic biliary duct cells and gallbladder cells expanded in vitro had differing phenotypes and gene expression profiles [18], which also suggest that some of the mAbs may be non-reactive to IHBT. Therefore, identification of the target antigens of GB Abs and future testing in IHBT could provide evidence of parallel or distinct biliary cell subsets within the liver.
The cross-reactivity of pancreatic ductal Abs in EHBT and GB Abs in ductal, exocrine, and endocrine pancreatic cells indicate that similar antigens are shared between pancreas and gallbladder/cystic duct cells. It is noteworthy that some gallbladder subsets highly express PDX1. PDX1 is known to be expressed in pancreatic endocrine and some duct cells and may identify a more primitive population [19]. Aside from the close developmental ontogeny with the pancreas, the functional significance of PDX1 expression in adult human EHBCs is not fully known [9, 11]. It can be speculated that these cells may be more amenable to genetic reprogramming towards the pancreatic endocrine and could be a target for regenerative medicine in diabetes [20, 21].
The antibodies may also have use in the diagnosis and study of gall bladder tumors. In general, the more intense labeling of apical mucosa of GBCA samples by GB1, GB3, and GB8 suggests that their antigens are upregulated in human gallbladder tumors, and are candidates to be tumor-associated antigens. Moreover, the apical membrane staining spilled into the lumen of GBCA sections, which indicates that the antigen may be shed or secreted into the lumen and could potentially find its way into the digestive tract. Whether this luminal-staining pattern, which was only evident in tumor tissues but not normal controls, has any promise for identifying gallbladder cancer biomarker(s) will depend on further extensive studies and identification of the antigens. Overall, our immunofluorescence studies on primary GBCA suggest that additional evaluation of the mAbs is warranted in other carcinomas of the GI tract.
Summary
The gallbladder cell subtype-marking antibodies described here have revealed antigenic and transcriptional heterogeneity within the human gallbladder and cystic duct. Heterogeneity within the epithelial cells of the human gallbladder and cystic duct was demonstrated by flow cytometric, immunofluorescence, and gene expression analyses. Currently the functional/physiological significance of these EHBC subsets remains unknown. In the future the ability to subdivide antigenically different epithelial cells of the gallbladder may be useful in dissecting the normal and pathologic physiology of this organ as it relates to bile transport, processing, stone formation, cellular plasticity, and cancer development.
Supplementary Material
Highlights.
Novel surface-reactive monoclonal antibodies (mAbs) were made from adult human gallbladder.
Antigenically distinct gallbladder subpopulations were isolated using these mAbs by FACS.
Three mAbs may be used to identify biomarkers for gallbladder carcinoma.
Acknowledgments
We thank Dr. Susan Orloff (Department of Abdominal Organ Transplantation/Hepatobiliary Surgery, OHSU), Dr. Christopher Corless, Leif Goranson, Angelica Jackman, Shannon Alcorta, and Kelly Kester (Department of Pathology) for human gallbladder tissue specimens. We thank Pamela Canaday (Flow Cytometry Core, OHSU) for FACS. We thank Aletha Lesch (Knight BioLibrary), Jenny Miller and Joscelyn Zarceno (Histopathology Shared Resources, OHSU) for archival sections of gallbladder carcinomas. This work was supported by the National Institutes of Health grant 5UO01DK089569-05 (Beta Cell Biology Consortium/M.G.) and The Leona M. and Harry B. Helmsley Charitable Trust grant 2012PG-T1D010 (M.G.)
ABBREVIATIONS LIST
- CCKAR
cholecystokinin A receptor
- EHBT
extrahepatic biliary tissue
- EHBC
extrahepatic biliary cell
- EPCAM
epithelial cell adhesion molecule
- GB
human gallbladder
- GBCA
gallbladder carcinoma
- HPd
human pancreatic duct
- KRT19
keratin 19
- LMNA
lamin A/C
- mAb
monoclonal antibody
- MUC5B
mucin 5B, oligomeric mucus/gel-forming
- MYH11
myosin, heavy chain 11, smooth muscle
- PBGs
Peribiliary glands
- PDX1
pancreatic and duodenal homeobox 1
- RAS
Rokitansky-Aschoff sinuses
- SOX9
SRY (sex determining region Y)-box 9
- VIM
vimentin
Footnotes
Conflict of Interest statement
OHSU has commercially licensed some of the technology described herein (HPd1/DHIC2-4A10 and HPd3/DHIC5-4D9); authors C.D., P.R.S and M.G. are inventors of these antibodies. This potential conflict of interest has been reviewed and managed by OHSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, Kaestner KH, Grompe M. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes & development. 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M. Surface markers for the murine oval cell response. Hepatology. 2008;48:1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9(+) liver progenitors in mouse oval cell injury. Hepatology. 2014;60:278–289. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME, Reid LM. Human hepatic stem cells from fetal and postnatal donors. The Journal of experimental medicine. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Manohar R, Komori J, Guzik L, Stolz DB, Chandran UR, LaFramboise WA, Lagasse E. Identification and expansion of a unique stem cell population from adult mouse gallbladder. Hepatology. 2011;54:1830–1841. doi: 10.1002/hep.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, Inverardi L, Dominguez-Bendala J, Ricordi C, Gerber D, Gaudio E, Alvaro D, Reid L. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, Alvaro D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology. 2012;55:2041–2042. doi: 10.1002/hep.25587. [DOI] [PubMed] [Google Scholar]
- 11.Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, Alvaro D, Reid LM, Gaudio E. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 13.Dorrell C, Abraham SL, Lanxon-Cookson KM, Canaday PS, Streeter PR, Grompe M. Isolation of major pancreatic cell types and long-term culture-initiating cells using novel human surface markers. Stem Cell Res. 2008;1:183–194. doi: 10.1016/j.scr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Long DJ, 2nd, Buggs C. Microwave oven-based technique for immunofluorescent staining of paraffin-embedded tissues. Journal of molecular histology. 2008;39:1–4. doi: 10.1007/s10735-007-9093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terada T. Histopathologic features and frequency of gall bladder lesions in consecutive 540 cholecystectomies. International journal of clinical and experimental pathology. 2013;6:91–96. [PMC free article] [PubMed] [Google Scholar]
- 16.Dorrell C, Schug J, Lin CF, Canaday PS, Fox AJ, Smirnova O, Bonnah R, Streeter PR, Stoeckert CJ, Jr, Kaestner KH, Grompe M. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54:2832–2844. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dianat N, Dubois-Pot-Schneider H, Steichen C, Desterke C, Leclerc P, Raveux A, Combettes L, Weber A, Corlu A, Dubart-Kupperschmitt A. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 2014;60:700–714. doi: 10.1002/hep.27165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manohar R, Li Y, Fohrer H, Guzik L, Stolz DB, Chandran UR, LaFramboise WA, Lagasse E. Identification of a candidate stem cell in human gallbladder. Stem Cell Res. 2015;14:258–269. doi: 10.1016/j.scr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, Bonner-Weir S. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci. 2010;123:2792–2802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey RD, Galivo F, Schug J, Brehm MA, Haft A, Wang Y, Benedetti E, Gu G, Magnuson MA, Shultz LD, Lagasse E, Greiner DL, Kaestner KH, Grompe M. Generation of islet-like cells from mouse gall bladder by direct ex vivo reprogramming. Stem Cell Res. 2013;11:503–515. doi: 10.1016/j.scr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coad RA, Dutton JR, Tosh D, Slack JM. Inhibition of Hes1 activity in gall bladder epithelial cells promotes insulin expression and glucose responsiveness. Biochem Cell Biol. 2009;87:975–987. doi: 10.1139/o09-063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.