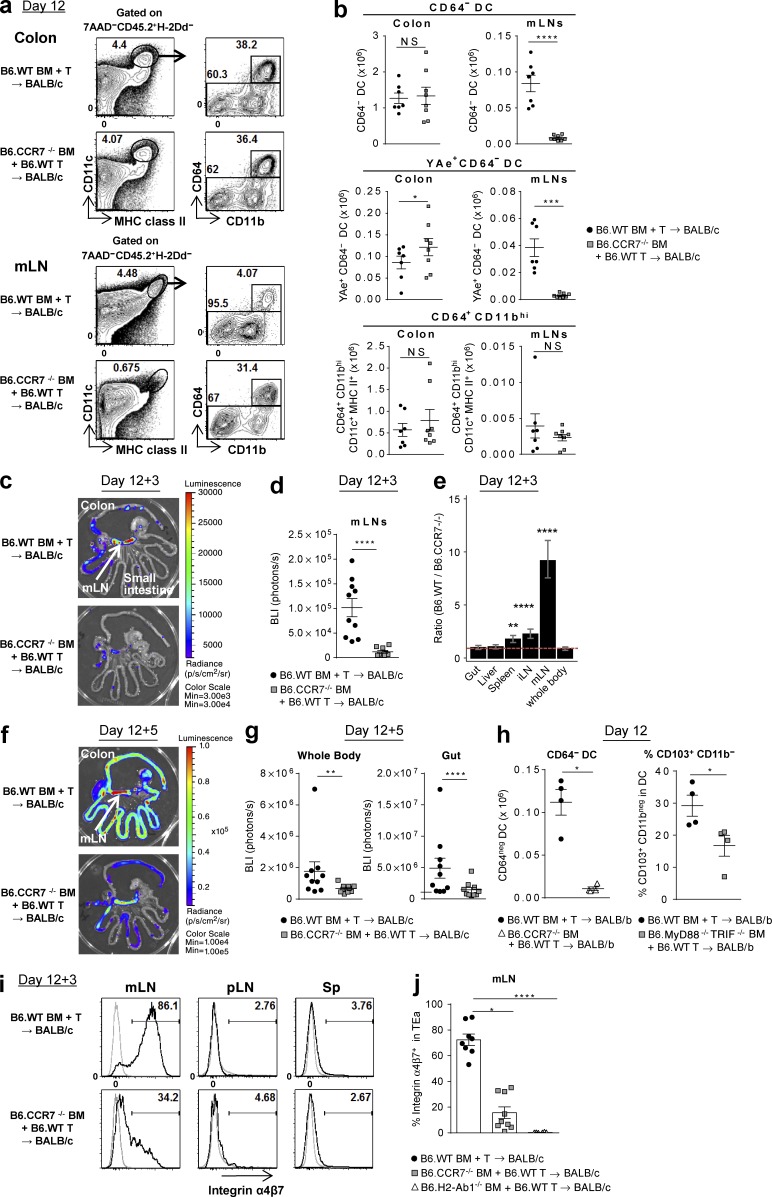
Figure 10.
Colon-derived donor DCs migrate to the mLNs via CCR7 and imprint the α4β7 gut-homing integrin on donor T cells. (a–g, i, and j) BALB/c mice were transplanted with TCD BM from B6.WT, B6.CCR7−/−, or B6.H2-Ab1−/− mice with B6.WT T cells. (a and b) Colon and mLNs were isolated on day 12. Representative FACS plots (a) and the absolute numbers of CD64− DC, YAe+CD64− DC, and CD64+CD11bhi subsets (b). Data shown are combined from three replicate experiments (n = 7–8 per group). ****, P < 0.0001; ***, P = 0.0003; *, P = 0.0413. (c–g, i, and j) On day 12 TEaluc+ T cells were injected, and 3 (c–e, i, and j) or 5 d (f and g) later BLI signals and cells from spleen, mLNs, and pLNs were analyzed. Representative BLI (c and f) and quantification of BLI signals (d and g). ****, P < 0.0001; **, P = 0.0012. (e) Ratio of BLI signals from B6.WT BM recipients to those from B6.CCR7−/− BM recipients by mixed-model analysis. ****, P < 0.0001; **, P = 0.00301 versus whole body. Data shown are combined from two replicate experiments (n = 9–10 per group). (h) BALB/b (H-2b) mice were transplanted with TCD BM from B6.WT, B6.CCR7−/−, or B6.MyD88−/−TRIF−/− mice with B6.WT T cells, and mLNs were isolated on day 12. The absolute numbers of CD64− DCs and the frequency of the CD103+CD11b− DC subset are shown. Data shown (n = 4 per group) are representative of two replicate experiments. *, P = 0.0286 (CD64− DC); *, P = 0.0367 (% CD103+CD11b−). (i and j) Representative FACS plots (i) and quantification (j) for α4β7 integrin expression, gated on TEa cells. Data shown are combined from two replicate experiments (n = 6–9 per group). ****, P < 0.0001; *, P = 0.0198. Data are represented as mean ± SEM.