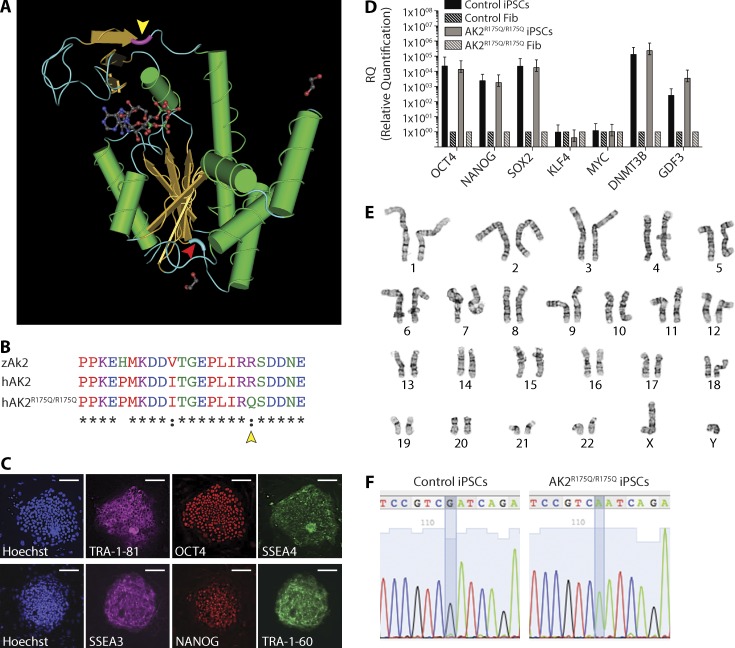
Figure 7.
Generation and characterization of human AK2R175Q/R175Q iPSCs. (A) Schematic representation of the annotated 3D structure of the human AK2 protein. The amino acid position mutated in patient-derived AK2R175Q/R175Q mutant fibroblasts and iPSC lines is highlighted in violet (yellow arrowhead). The amino acid position mutated in ak2L124P/L124P mutant zebrafish is marked in light blue (red arrowhead). (B) Partial sequence multi-alignment of the LID domain of zebrafish (zAk2) and human (hAK2) proteins and the human mutated form (hAK2R175Q/R175Q). Yellow arrowhead marks the amino acid position mutated in patient-derived cell lines. (C–F) iPSCs were generated from AK2-deficient dermal and control foreskin fibroblasts. (C) Representative confocal microscopy images showing the expression of human pluripotency markers (TRA-1-81 and SSEA3 [magenta], Oct4 and NANOG [red], and SSEA4 and TRA-1-60 [green]) using immunofluorescently labeled antibodies in AK2R175Q/R175Q iPSCs; cellular content is highlighted by nuclear staining with Hoechst 33342 (blue). Bars, 100 µm. (D) mRNA analysis using quantitative real-time PCR of the indicated pluripotency-associated genes in AK2R175Q/R175Q and control iPSCs. AK2R175Q/R175Q and control iPSCs gene expression was compared with the respective parental fibroblasts (Fib), and human β-actin gene expression (hACTB) was used as housekeeping gene. Error bars indicate standard error. (E) Karyotype and G-banding analysis of AK2R175Q/R175Q iPSCs. (F) Sequencing of the genomic region surrounding the mutation in the control and patient-derived AK2-mutated iPSC line. Data (C–F) are representative of at least two independent experiments.