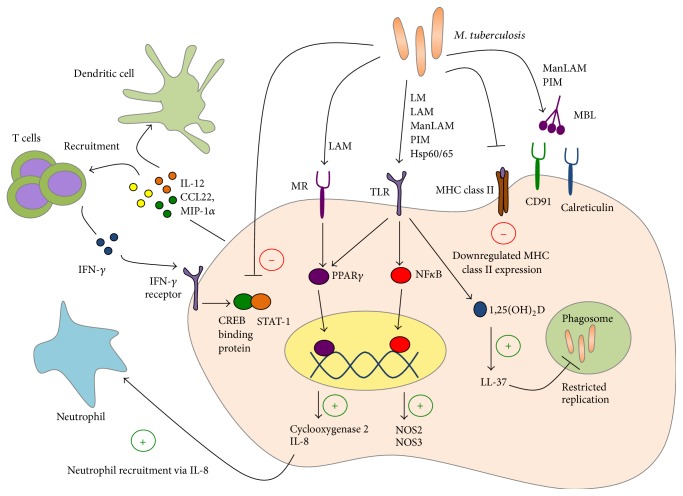
Figure 1.
Human alveolar macrophages possess an array of receptors to recognize M. tuberculosis (Mtb). In response to Mtb infection, macrophages upregulate effector and signalling pathways to both prevent bacterial replication and recruit other immune cells into the site of infection. M. tuberculosis components, including lipoarabinomannans (LAMs), lipomannans (LMs), phosphatidylinositol mannosides (PIMs), and heat shock proteins (HSPs), are recognized by a variety of pattern recognition receptors. Following recognition of Mtb, host effectors, such as NF-κB and PPARγ, are activated to upregulate antimicrobial factors. These antimicrobial peptides (e.g., LL-37) possess both effector and signalling functions to actively interfere with bacterial replication as well as recruit and activate neutrophils, dendritic cells, and T cells. However, Mtb interferes with macrophage effector and signalling pathways. Most importantly, Mtb downregulates MHCII expression on macrophages to prevent optimal interaction with antigen specific T-cells. Furthermore, Mtb interferes with IFNγ signaling, a T cell cytokine mediator critical for upregulating the inherent antimicrobial capacity of macrophages during infection.