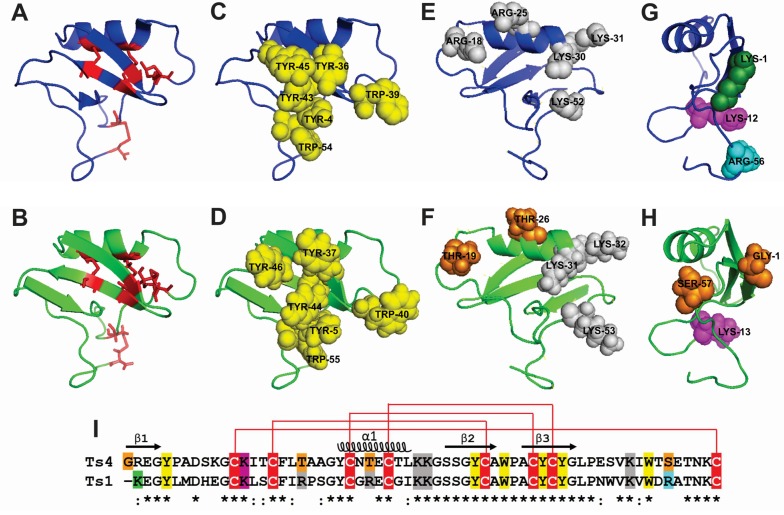
Figure 4.
Cartoon representation of Ts1 structure and Ts4 3D-structural model. The cartoons represent the flattened triangular shape structure of the α-helix/β-sheet motif (CSαβ) of Ts1 (blue) and Ts4 (green). The Ts4 different amino acid residues compared to Ts1 are highlighted in orange. (A,B) Face A (front view): Cysteine residues are shown as red stick. (C,D) Face A (front view): Conserved aromatic cluster (yellow). (E,F) Face B (back view): Important structural positive residues (grey). (G,H) Face C (side view): Residues implicated in voltage-gated sodium channel (Nav) activity. N-terminal amino acid residue (green). Residue implicated in β-toxin activity (magenta). Residue implicated in α-toxin activity (cyan). (I) Sequence alignment of Ts4 and Ts1. The alignment of the Ts4 and Ts1 were created by Clustal Omega version 2.1. (*) identical residues; (:) highly conserved residues. Cysteine residues are highlighted in red. The residues are following (A–H) coloring patterns.