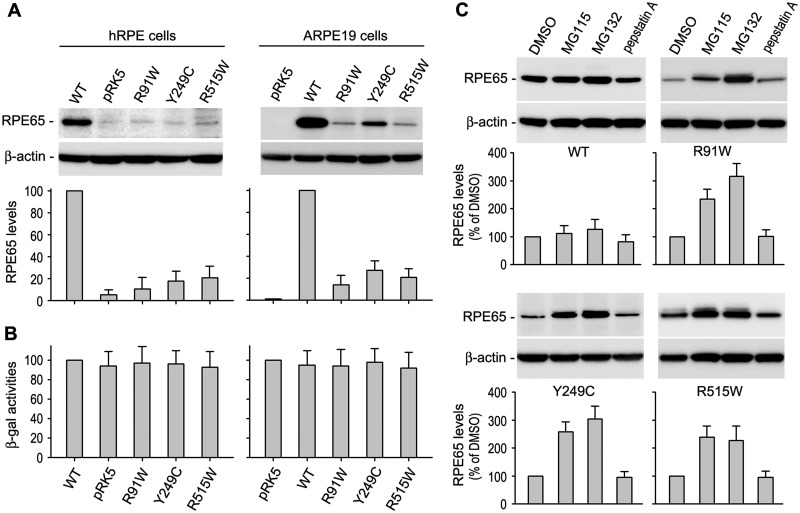
Fig. 1.
Mutant RPE65 proteins are degraded in the proteasome. (A) Immunoblot analysis of RPE65 in primary human RPE (hRPE) and ARPE-19 cells transfected with pRK5 mock vector or construct encoding wild-type (WT) or mutant (R91W, Y249C and R515W) RPE65. Beta-actin was detected as a loading control. Relative intensities of the RPE65 immunoblots were quantified, normalized by the β-actin levels, and expressed as percentage of WT RPE65. (B) Relative β-gal activities from pRSV-LacZ co-transfected with RPE65 constructs into the cells. (C) Immunoblot analysis of WT and the mutant RPE65s in ARPE-19 cells treated with inhibitors of the proteasome (MG115 and MG132) or lysosome (pepstatin A). Relative intensities of the immunoblots were quantified and expressed as percentage of RPE65 in the cells treated with DMSO. All error bars show SD (n = 3).