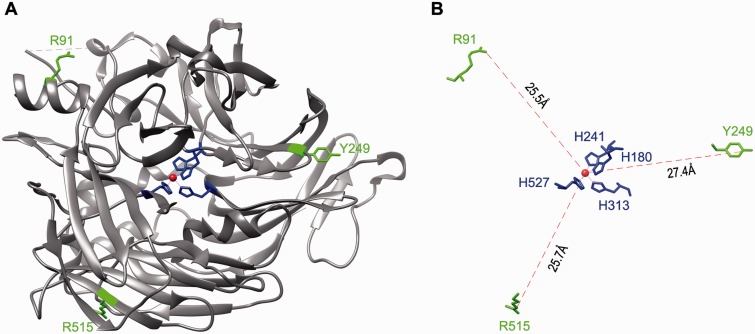
Fig. 4.
Mapping of the three mutation sites on the crystal structure of RPE65. (A) A three-dimensional image of the crystal structure of the bovine RPE65. The catalytic site containing Fe2+ (brown sphere) and four iron-binding histidine residues is in the center of RPE65 structure. The three mutation sites (R91, Y249 and R515) shown in green are mapped in the non-active sites of RPE65. (B) A geometry showing the distances from the iron ion to the mutation sites. H180, H241, H313 and H527 are iron-binding histidine residues.