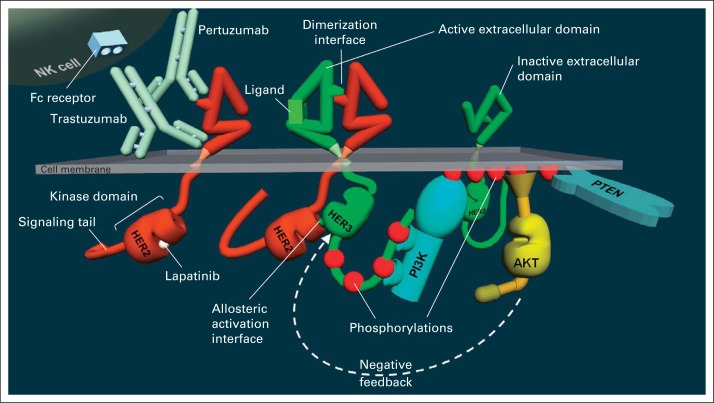
Fig 1.
Structure of the human epidermal growth factor receptor 2 (HER2) and HER3 receptors and their mode of activation through dimerization and activation of PI3K/Akt signaling and binding sites of trastuzumab, pertuzumab, and lapatinib, showing both an inactive and ligand-activated HER3. Binding of ligand reconfigures the extracellular domain of HER3, exposing the dimerization interface. The extracellular domain of HER2 is always in the active configuration and does not require ligand. The phosphorylated signaling tail of HER3 binds and activates PI3K, leading to phosphorylation of membrane lipids, which is reversed by the phosphatase PTEN. These membrane phospholipids recruit and activate Akt, which regulates many downstream events. In HER2-driven cancer cells, it also regulates HER3 in a feedback loop shown by the arrow.