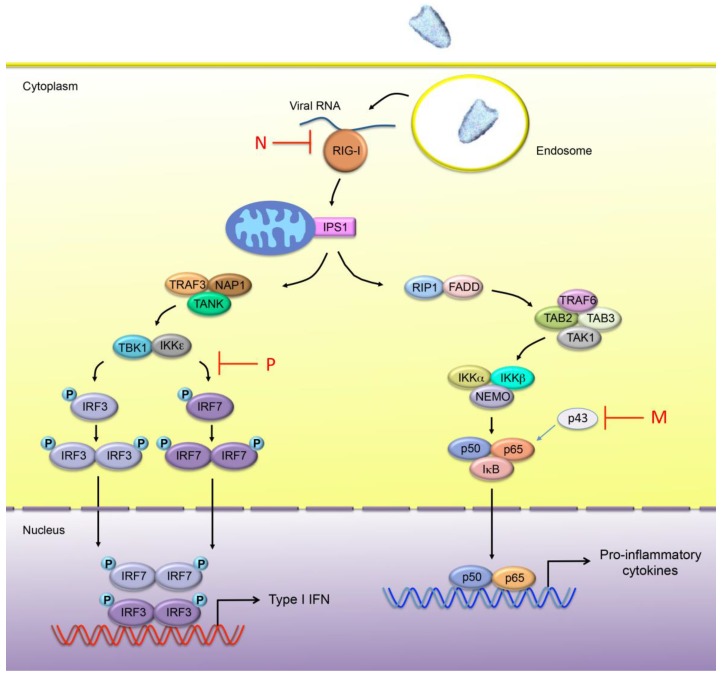
Figure 2.
Innate immune sensing of rhabdovirus infection. In rhabdovirus-infected cells, the viral RNA is mainly detected by RIG-I. Once activated, RIG-I binds to the CARD containing adaptor protein IPS-1 (also known as MAVS, CARDIF or VISA), which phosphorylates IRF3 and/or IRF7 through TRAF3, NAP1 and TBK1/IKKε. Phosphorylated IRF3 and IRF7 homodimerize and translocate into the nucleus where they induce the expression of Type I IFN genes. IPS-1 also interacts with FADD, a death domain-containing adapter involved in death receptor signaling, and RIP1, which induces the activation of the NF-κB pathway. NF-κB is composed of homo- and heterodimeric complexes of members of the Rel family. The most common and best-characterized form of NF-κB is the p65/p50 heterodimer. A new member RelAp43 (p43) of the NF-κB family has been recently identified. Activation of the IκB kinase (IKK) complex, consisting of catalytic kinase subunits (IKKα and/or IKKβ) and the regulatory non-enzymatic scaffold protein NEMO, results in the phosphorylation and subsequence degradation of IκB. This enables free NF-κB to translocate to the nucleus, where it induces target gene expression, including pro-inflammatory cytokine encoding genes. The steps inhibited by the RABV-N and -P proteins, as well as VSV M protein are indicated in the diagram above.