Scheme 4.
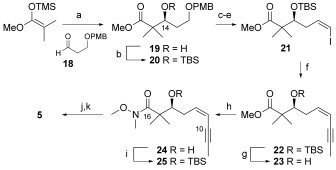
Synthesis of the C10–C19 β-hydroxy ketone fragment 3. Reagents and conditions: a) 1. N-Ts-D-Valine, BH3⋅THF, CH2Cl2, −78 °C, 5 h, 2. HCl, THF:H2O (1:1), 85 %, 89 % ee; b) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C, 2.5 h, 94 %; c) DDQ, CH2Cl2:H2O (18:1), RT, 1.5 h, quant.; d) Swern, 95 %; e) ICH2PPh3+I−, NaHMDS, HMPA, THF, −78 °C, 2 h, 75 %; f) 1. BrMgC≡CCH3, ZnCl2, THF, 0 °C, 30 min, 2. 20, [PdCl2(PPh3)2], 0 °C to RT, 16 h, 92 %; g) HF (40 % aq.), MeCN, 0 °C to RT, 1 h, 99 %; h) 1. HNMe(OMe)⋅HCl, nBuLi, −78 °C to RT, 30 min, 2. 22, THF:hexane (1:1), −78 °C to RT, 1.5 h, 82 %; i) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C to RT, 2 h, 96 %; j) 1. allyl-MgBr, Et2O −20 to −78 °C, 2 h, 2. DBU, Et3N, 50 °C, 18 h, 81 %; k) HF (40 % aq.), MeCN, 0 °C to RT, 45 min, 85 %.
