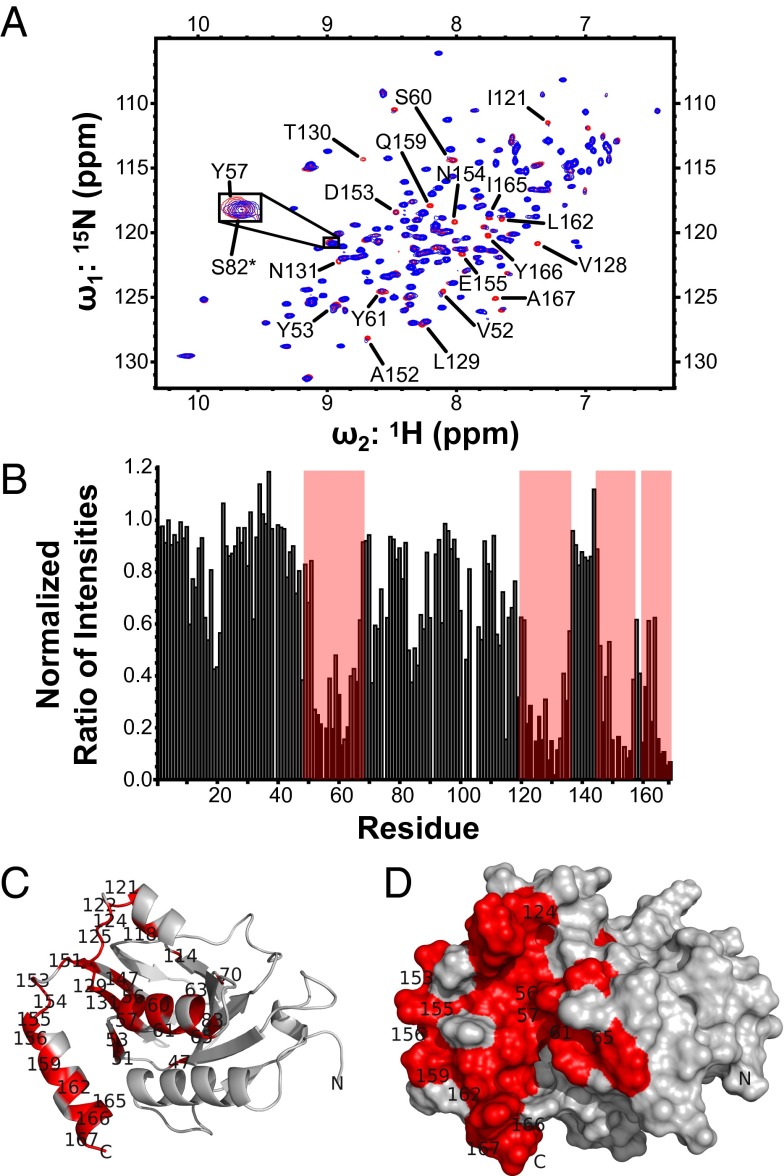
Fig. 2.
UL53 peptide binding site. (A) Heteronuclear single-quantum coherence spectrum of 15N M50 (1–168) with equimolar unlabeled UL53 peptide titrated into the sample. The expanded view shows Y57 as an example of a peak that shows chemical shift perturbation on the addition of the peptide and S82 (marked with an asterisk) as an example of a peak that shows no chemical shift perturbation. (B) Bar graph of the normalized ratio of peak intensities with equimolar unlabeled UL53 peptide present to peak intensities with no unlabeled UL53 peptide present for each residue. Areas highlighted by red boxes are residues that show marked reduction in peak intensity on addition of the peptide, and these areas correspond to the residues shown in red in C and D. (C) Ribbon representation of M50 (1–168), with residues showing a nonzero normalized ratio of peak intensity lower than 0.4 in titration experiments highlighted in red. (D) Surface representation of M50 (1–168), with residues showing a nonzero normalized ratio of peak intensity lower than 0.4 in titration experiments highlighted in red. Only a subset of residues has been labeled in C and D for clarity of presentation.