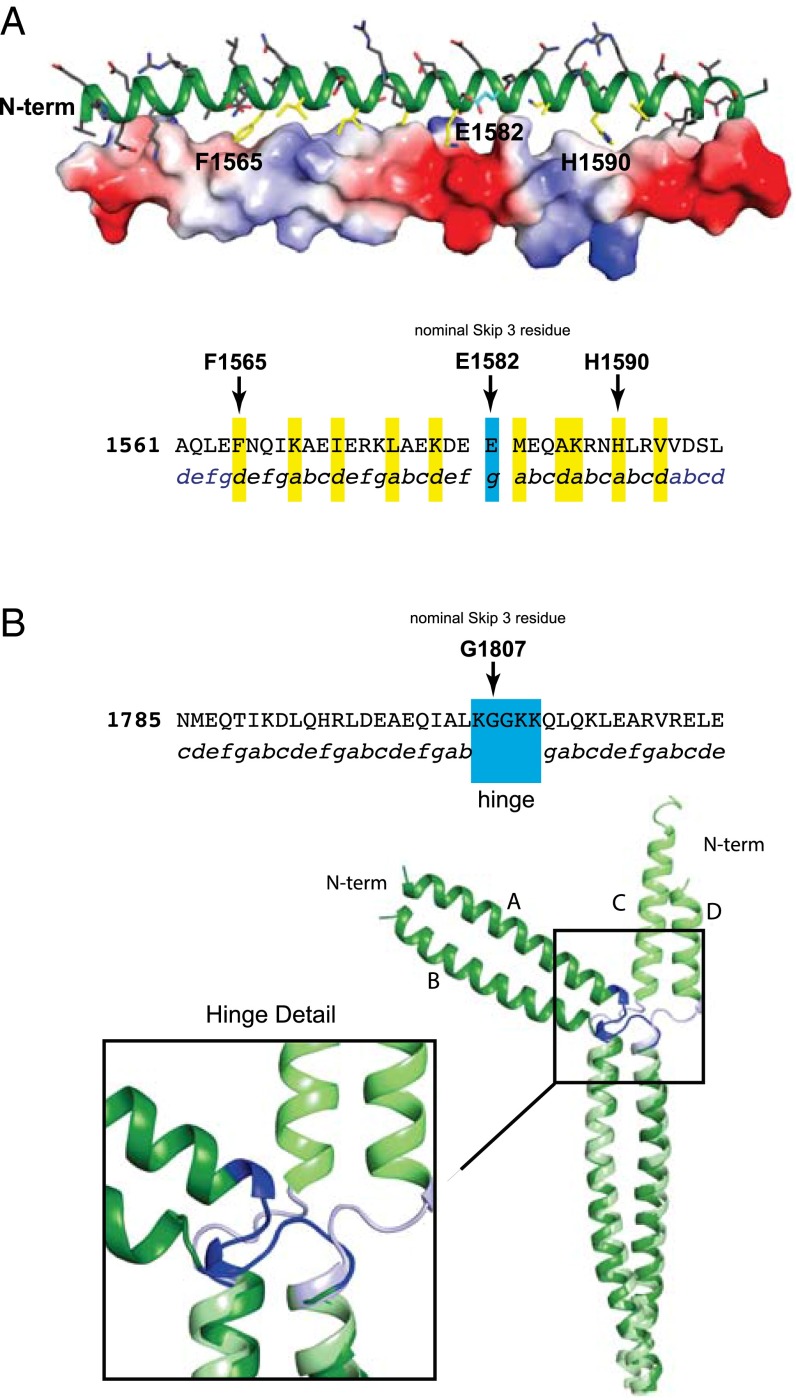
Fig. 2.
Structural analysis of Skip 3 and Skip 4. (A, Upper) A cartoon representation of the coiled-coil surrounding E1582 (Skip 3) and with a surface electrostatic representation shown on the lower-helix. (Lower) The protein sequence surrounding Skip 3. Packing residues in the nonconical coiled-coil region of the upper-helix are colored in yellow on the upper-helix. The skip residue is colored in cyan. The protein sequence surrounding the nominal skip residue, E1582, with the observed coiled-coil position registry shown below. Residues that are in a standard packing arrangement are in blue and the atypical region is colored black. Residues packing along the distorted interface are highlighted in yellow and the skip residue is in cyan. (B) The sequence and structure of Skip 4. The protein sequence surrounding the nominal Skip 4 residue, G1807, is shown with the coiled-coil position registry below. Residues involved in the hinge region are indicated. A C-terminal structural alignment of residues Q1811–T1854 in chains A and B superimposed on C and D for the two independent molecules in the asymmetric unit for Skip 4 is also shown. This reveals the conformational variability in the Skip 4 hinge. The stabilization and folding domains were omitted from all structural figures.