Abstract
Objective:
Due to the structure and physiological barrier of eye, only 1% of instilled dose is available for action on the corneal surface. In this work, we developed and evaluated chitosan (pH sensitive) and gellan gum (ion sensitive) in situ gel of sparfloxacin to improve precorneal residence time.
Materials and Methods:
A protocol for radiolabeling of sparfloxacin with Tc-99m was optimized to study the ocular retention using gamma scintigraphy technique.
Results:
The clear formulation was developed. In vitro release showed a sustained and prolonged release compared to plain eye drop solution. Dynamic and static gamma scintigraphy showed better retention than plain eye drops. The ocular tolerance test (hen's egg test-chorioallantoic membrane test and infra-red study) showed that the formulation is nonirritant and can be used as ocular vehicle.
Conclusion:
Radiolabel protocol for sparfloxacin was successfully developed and evaluated on ocular retention studies of developed in situ gel. The developed in situ gel is non irritant and can go further with clinical evaluation.
KEY WORDS: Gamma scintigraphy, in situ gel, ocular delivery, sparfloxacin
Human eye is a complex structure that provides unique challenges for ocular drug delivery. In comparison to another route of administration, most of the drugs are impermeable to eye and the instilled conventional eye drops drained from the eye through the nasolacrimal duct. The major problem encountered in ocular drug delivery is the attainment of an optimum drug concentration at ocular surface. Poor bioavailability of actives from conventional eye drops depends on various factors including precorneal loss factors that is, rapid tear turnover, nonproductive absorption, transient residence time and the relative impermeability of the drugs to corneal epithelial membrane. These factors made ocular drug delivery a challenging problem. Normally, ocular diseases like dry eye syndrome, glaucoma, and conjunctivitis, etc., require frequent drug administration. Sometimes systemic absorption of the drug drained through the nasolacrimal duct may result in some undesirable side effects.[1,2,3]
Due to these physiological and anatomical constraints, clinician recommends a frequent dosing at an extremely high concentration and pulse type dosing results in several side effects. Novel ocular drug delivery systems offer some improvement over a conventional liquid dosage form but because of lack of patient compliance that is, blurred vision (e.g., ointments),[4,5] surgical application (inserts) etc., they have not been universally accepted.[6,7] These problems may be overcome by the use of in situ gel-forming systems that are instilled as drops into the eye and undergo a sol-gel transition in the cul-de-sac.[8]
In the present preliminary work, a protocol for radiolabeling of sparfloxacin was developed and the combination of chitosan and gellan gum is evaluated as in situ gel system with sparfloxacin as drug. Radiolabeled formulation is evaluated for precorneal retention and lacrimal clearance by gamma scintigraphy[9,10] along with its ocular tolerance. Usually, antibiotics are prescribed for ocular infections with 4–5 times in a day mainly because of low retention time on the ocular surface. Here we are trying to enhance ocular retention to reduce drug frequency.
Materials and Methods
Sparfloxacin was received as a kind gift from Micro Labs Ltd., (Chandigarh, India). Chitosan (practical grade, 75–85% deacetylated, molecular weight 150 kDa) was obtained as a kind gift from M/s, India Sea Foods, India. Gellan gum (Gelrite® CP Kelco, USA) was obtained as a gift sample from Applied Biosciences, Mumbai, India. All other chemicals and solvents used were purchased from local suppliers and of analytical grade unless mentioned.
Labeling of sparfloxacin with technetium-99m
Due to a short half-life and other benefits, we used technetium-99m (Tc-99m) as radionuclide for radiolabeling of sparfloxacin. Tc-99m was obtained as sodium pertechnetate in normal saline eluted from molybdenum generator from regional center of (Board of Radiation and Isotopes Technology), Institute of Nuclear Medicine and Allied Science (INMAS), Delhi, India. For labeling a 1.5 mg of the drug was dissolved in 2 drops of 1M NaOH and mixed with stannous chloride (SnCl2 1 mg/ml in 10% acetic acid). To this Tc-99m (2–3 mci) was added and mixed properly. All the radiolabelling operation was carried out in a hot lab under lead shielding.
Determination of labeling efficiency
Labeling efficiency was checked using Instant Thin Layer Chromatography (ITLC) technique. A drop of the radiolabeled formulation was applied on to ITLC silica gel coated strips (Gelman Science Inc., Ann Arbor, MI), which were run in 100% acetone as mobile phase. After the acetone reach just below the top, then the strips were taken out and dried. This strip was then cut into two equal halves, and each half was cut into small pieces and radioactivity was counted using well-type gamma counter (CAPRAC-R, Capintec, USA). Labeling efficiency was calculated from the following formulae.
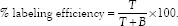
Where: T = counts at top, B = counts at bottom
The labeled formulation sometimes contains reduced/hydrolyzed Tc-99m (colloids) which could also chromatograph as bound. Hence to separate them, another mobile phase (PAW) with similar stationary phase (ITLC strips) was used. PAW consisted of pyridine: Acetic acid: Water in 3:5:1.5 ratio respectively. In this phase only reduced/hydrolyzed Tc remained at the point of origin while labeled complex will move along with a solvent front. Percent colloids were calculated using the following formula.
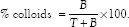
Optimization of labeling conditions (pH, SnCl2 concentration)
Labeling efficiency of the drug molecules generally depends on the reducing agent (SnCl2 concentration) and the pH. Hence to achieve maximum radiolabelling efficiency, labelling experiments were carried out using different concentration of SnCl2 and varying pH (by adding 0.5 M NaHCO3 solution) by keeping pH and reducing agent concentrations constant respectively. Labeling efficiency was calculated as per formula above and reported as % labeling efficiency and % colloids. Data was represented as the average (mean) of five individual readings and its standard deviation (SD).
In vitro stability of labeled complex
To ensure the stability of radiolabeled drug complex for entire period of time of experiment, a 100 μL aliquot of the labeled formulation was mixed with 2.0 mL of phosphate-buffered saline (pH 7.4) and incubated at room temperature for a period of 24 h. Labeling efficiency of the drug – Tc-99m complex was measured in between to ensure stable complex.
Preparation of in situ gel formulation
Different combinations of placebo formulations were developed and screened for viability [Table 1]. Chitosan (viscosity 175 cps, insolubles 0.20%, deacetylation 84%, pH 8.34, moisture 7%, ash 0.48%, total plate count <5000/G) was dissolved in 1% acetic acid and pH adjusted to 5.5–6.0 by phosphate buffer system. Gellan gum was dissolved in phosphate-buffered saline, pH 7.4. Viscosity was measured using Brookfield's viscometer (model DV II, spindle no. 02, at 20 rpm), and clarity was examined by visual inspection. Different combinations were tested, and the best combination is chosen for further developing medicated in situ gel. The formulations were selected/rejected on the basis of their viscosity, clarity, and turbidity with change in pH. Sparfloxacin is available as 0.5% eye drops concentration for antibacterial activity. Hence, a dose of 0.5% sparfloxacin was used in in situ gel formulation for proper comparison. The weighed quantity of drug was placed in volumetric flask and dissolved primarily in 1–2 drops of ethanol and then volume was made up in normal saline under aseptic conditions. 0.1% of methyl paraben is approved for ocular use, so same was added as a preservative.[11] The resultant solutions were mixed well and kept undisturbed at room temperature for 24 h. Osmolarity of the formulation was determined by osmometer (Fiske Associate, USA) and required amount of sodium chloride was added and mixed thoroughly to make the solution isotonic. Complete formula of the developed formulation is given in Table 2. The other physicochemical evaluation parameters that is, clarity, gelation pH, osmolarity etc., were also measured as per previously available literature.[8,12] The plain drug solution of drug was prepared in the same manner removing polymers (chitosan and gellan gum) and make it isotonic.
Table 1.
Different combinations evaluated for placebo in situ hydrogel formulation of sparfloxacin
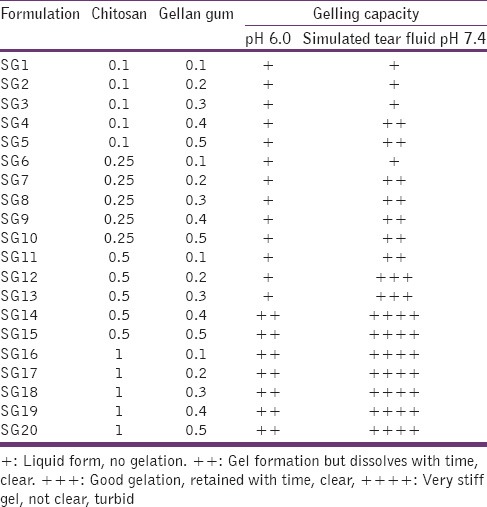
Table 2.
Ingredients of medicated formulation

In vitro drug release profile
In vitro release was determined by modified dialysis membrane technique. A 1 ml aliquots of the formulation and marketed formulation were taken in the dialysis tube (Sigma Chemicals, USA), which was suspended in separate beakers. Care was taken to make sure that no air bubbles were entrapped inside the polymer solutions. The beakers were then filled with 100 ml of simulated tear fluid, pH 7.4 and placed in release medium with a circulating water bath equipped with a stirrer to stir continuously. The temperature and stirring rate were maintained at 37°C ± 0.5°C at 75 rpm. Aliquots of 1 ml were withdrawn from the release medium at different time intervals and equal volumes of fresh media were added to replace the withdrawn samples. Withdrawn samples were filtered and diluted appropriately. Prepared samples were estimated for the drug content by ultraviolet spectrophotometry at 290 nm. Cumulative percent drug released was calculated. Results are shown in Figure 1.
Figure 1.
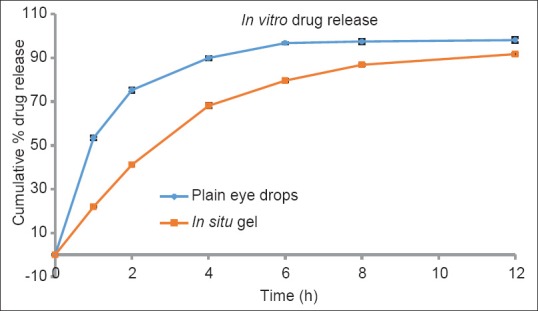
In vitro drug release profile of sparfloxacin (blue) plain eye drops (orange) In situ gel
Ocular irritation test (hen's egg test-chorioallantoic membrane test)
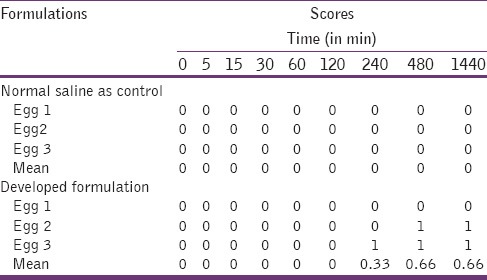
For the present study, modified hen's egg test-chorioallantoic membrane test (HET-CAM) test as reported by our group before was carried out.[8] Briefly, fertilized hen's eggs weighing between 50 and 60 g were obtained from the poultry farm. These eggs were candled in order to discard the defective ones. These selected eggs were incubated in humidified incubator at a temperature of 37°C ± 0.5°C for 3 days. The eggs were rotated manually in a gentle manner after every 12 h. On the 3rd day, 3 ml of the egg albumin was removed using sterile techniques from the pointed end of the egg. After removing the albumin, the hole was sealed by parafilm (American Can Company, USA). The eggs were kept in the equatorial position for the development of CAM away from the shell. On the 5th day of incubation, the eggs were candled and checked every day thereafter. The nonviable embryos were removed. On the 10th day of incubation, a window (2 cm × 2 cm) was made on the equator of the eggs and the 0.5 ml of the formulations were instilled directly onto the CAM surface and left in contact for 5 min. The membrane is then examined for vascular damage and the time taken for injury to occur is also recorded. As a control solution, a 0.9% NaCl solution was used. The scores were recorded according to the scoring schemes as shown in Table 3 and score obtained is given in Table 4.
Table 3.
Scoring chart for HET-CAM test
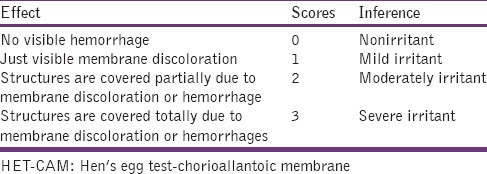
Table 4.
Scores obtained in HET-CAM test
Ocular inflammation (infra-red camera)
Ocular inflammation/tolerance was also evaluated using hand held infra-red (IR) camera. It is based on the principle that if there is any inflammation in the organ then there will be an increase in surface temperature. The temperature of the ocular surface was noted using hand operated IR camera before and after application of the formulation into rabbit eye.
Gamma scintigraphy
Retention efficacy of the developed in situ gel was evaluated by gamma scintigraphy. Albino rabbits weighing 2–3 kg were used for this study. Animals were procured from the animal house of INMAS (Delhi, India). The study was carried out under the guidelines compiled by CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Culture, Govt. of India) and all the study protocols were approved by local Institutional Animal Ethics committee of INMAS. Utmost care was taken to ensure while working with animals. Weighed amount of sparfloxacin was dissolved in 1–2 drops of acetone and radiolabeled with Tc-99m as per the optimized protocol developed above. This radiolabelled drug solution was then mixed with other ingredients in such a way that the final solution would contain 0.5% w/v sparfloxacin and required concentration of polymers. Gamma camera (Millenium VG, USA), autotuned to detect the 140 KeV radiation of Tc-99m was used for capturing scintigraphic images. Rabbits were anaesthetized using ketamine HCl injection (15 mg/kg body weight) given intramuscularly and were positioned 5 cm in front of the probe. The 25 μl of the radiolabelled formulation (100 μci) was instilled onto the left corneal surface of the rabbits. Recording was started and continued for 30 min using 128 × 128 pixel matrix. Individual 60 frames (60 s × 30 s) were captured by time activity curve using dynamic imaging. Region of interest was selected on the one frame of the image and time-activity curve was plotted to calculate the rate of drainage from the eye. After dynamic readings, a single whole body static image was also taken after 5 h of instillation of formulations.
Results and Discussion
Ocular drug delivery or achieving prolonged retention on the ocular surface is a challenging task. In situ gel emerged out as an effective solution to ocular retention problem. Here, we demonstrated an in situ gel system of sparfloxacin using chitosan (pH activated) and gellan gum (ion activated) polymers. We successfully developed the formulation and evaluated for various physicochemical parameters. Evaluation of the ocular retention is also a challenge. Gamma scintigraphy is a technique whereby the transit of a dosage form through its intended site of delivery can be noninvasively imaged in vivo by the application of an appropriate short-lived gamma emitting radioisotope. It provides a good insight of formulation. In the present work, we have used pharmacoscintigraphy as a noninvasive tool in the evaluation of our developed in situ formulation. Drug was labeled with radionuclide Tc-99m. It was chosen for the purpose because of its moderate half-life (6 h). Further it emits gamma rays, which have relatively low energy as compared to α and β rays, so leads to no serious health hazards to the workers. Drug was instantaneously labeled with Tc-99m. Labeling efficiency was checked by ITLC using 100% acetone as mobile phase. The Rf value of free Tc is approximately 0.9, so it reached the top of the ITLC strip while due to the difference in molecular weight, the drug-Tc complex, retained at the base of ITLC strip. So from the difference in the top and bottom counts of the ITLC strips labeling efficiency is calculated. The labeling procedure also leads to the formation of reduced/hydrolysed Tc (colloids). To detect these colloids, ITLC was run in another solvent system PAW. Due to heavy weight, colloids were retained at the base while drug-Tc complex travelled to the top of the ITLC strip.
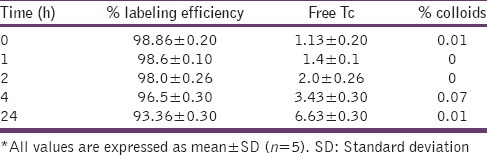
Various labeling parameters, e.g., SnCl2 concentration [Table 5] and pH [Table 6] were optimized and it was observed that at 50 μg SnCl2 concentration and at pH 7.0 gave maximum labeling efficiency (<98.5%). At these conditions minimum colloids (>1.1%) were produced. In vitro stability of the labeled complex was also checked, and the complex was found to be stable for up to 24 h with labeling efficiency of 93.36% [Table 7].
Table 5.
Effect of SnCl2 on labeling efficiency of sparfloxacin
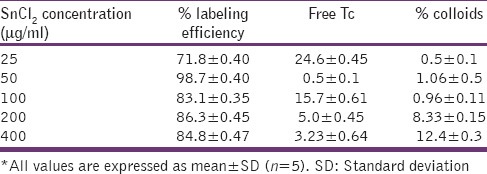
Table 6.
Effect of pH on labeling efficiency of sparfloxacin
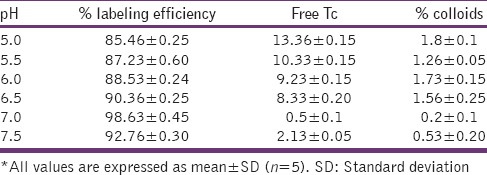
Table 7.
In vitro stability of sparfloxacin labeled formulation
Formulation development
The placebo formulations were developed using a different combination of chitosan and gellan gum which were evaluated for various physicochemical characteristics like gelling capacity, physical appearance and viscosity. As an ocular formulation, it's desired for the formulation to be clear with appropriate viscosity. Among different combination of polymers at formulation pH (pH 6.0) and physiological pH (pH 7.4) [Table 1], a combination of 0.5% of chitosan and 0.2% of gellan gum was selected as it provide colorless and transparent formulation with acceptable viscosity.
A medicated formulation was prepared from the selected placebo formulation. A dose of 0.5% of sparfloxacin is prescribed for conjunctivitis hence, we also used the same in the present formulation. 0.1% methyl paraben was added as preservative and NaCl in a calculated amount was also added to maintain isotonicity of the final formulation [Table 2]. The results for the physicochemical properties of the formulation are given in Table 8. An optimized iso-osmotic in situ gel formulation is developed and evaluated further.
Table 8.
Physicochemical properties of medicated in situ gel

In vitro drug release profile of the formulation was determined in S.T.F (pH 7.4) and the formulation demonstrates a slow release rate. Developed formulation shows 41.17% cumulative drug release after 2 h, 79.6% after 6 h and 91.7% after 12 h [Figure 1] whereas the plain eye drops released 75.33% in 2 h and 89.93% in 4 h. This shows that in situ gel release slowly than plain eye drops and presented a sustained and prolong the action.
Ocular tolerance
Ocular irritation of the developed formulation was checked by hen's egg CAM test. This is an inexpensive, rapid, and sensitive test. The HET-CAM has been shown to be a qualitative method of assessing the potential irritancy of chemicals which can be detected by observing adverse changes that occur in the chorionallantoic membrane of the egg after exposure to test chemicals.[13] The CAM of the chick embryo is a complete tissue including veins, arteries and capillaries and is technically very easy to study. It responds to injury with a complete inflammatory process similar to that induced in the conjunctival tissue of the rabbit eyes.[14] As the testing with incubated eggs is a borderline case between in vivo and in vitro systems so it does not conflict with the ethical and legal obligations.[15,16] Developed formulation was tested by this score chart given in Table 3 and result was compared with those obtained using normal saline, which was used as control (supposed to be practically nonirritant). A means score of 0 was obtained for normal saline. In situ gel-based formulation was nonirritant up to 2 h (mean score 0) while the mean score was found to be 0.33 in 4 h and reached 0.66 up to 24 h [Table 4]. The study shows that the formulation is nonirritant to mild irritant (score < 1) and is well tolerated.
Infrared camera
The observations of IR camera showed a slight increase in the temperature of the corneal surface upon application of the formulation that was considered quite normal due to reflex action of the eye. The reading did not show a rapid increase in corneal temperature before and after formulation application, hence no acute or chronic inflammation was depicted to the eye. The formulations were said to be well tolerable and safe to use [Table 9].
Table 9.
IR camera readings for sparfloxacin formulations
Gamma scintigraphy
Scintigraphic studies were conducted on Albino New Zealand rabbits using Tc-99m labeled sparfloxacin in the formulation. The gamma camera start capturing the images once we instilled the eye drops on the rabbit corneal surface. The half-life of Tc-99m is 6 h so we took a static image at 5 h. The observation of the acquired gamma camera images showed good spreading over the entire precorneal area for developed in situ gel [Figure 2b] immediately after administration as compared with plain drug solution [Figure 2a]. The plain drug solution eliminated quickly and distributed in body systemic circulation as seen in static images after 5 h [Figure 2]. The curve of the remaining activity on the corneal surface as a function of time (time-activity curve) is generated in Figure 3. There is a quick fall in % radioactivity remained on cornea for plain drug solution than for developed in situ gel. This implies that the developed in situ gel-based formulation was cleared at slow rate and retained at corneal surface for longer duration. No significant radioactivity was observed in systemic circulation (kidney and bladder), [Figure 2b]. Chitosan is both viscous and bioadhesive. Further, viscosity of chitosan is increased as the increase in pH from 6 (formulation pH) to 7 (physiological pH) upon instillation into eye as a result of buffering action of the tear fluid. Gellan gum also imparts viscosity on coming in contact with ions present in tear buffer of eye. This combination leads the formulation to stay for a longer time on the ocular surface that is easily detected by gamma scintigraphy technique.
Figure 2.
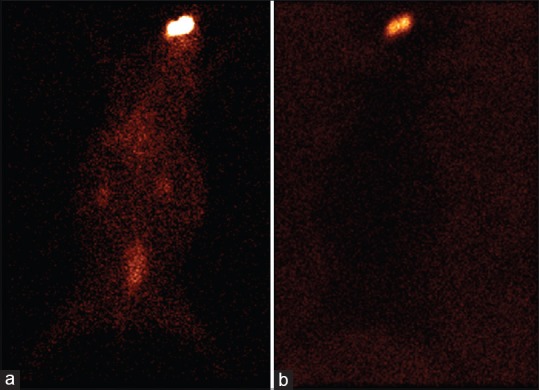
Gamma scintigraphy static images of rabbits after 5 h of technetium-99m labeled sparfloxacin formulation instillation (a) Plain eye drop formulation (b) Developed in situ gel formulation
Figure 3.
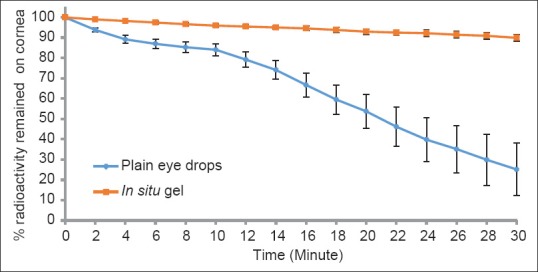
Gamma scintigraphy dynamic study (blue circle) plain eye drop formulation (red square) developed in situ gel formulation of sparfloxacin
Conclusion
Sparfloxacin drug was successfully radiolabeled with Tc-99m for subsequent evaluating the efficacy of the developed in situ gel system by pharmacoscintigraphy. This noninvasive technique has proved to be an important tool in evaluating ocular drug delivery system especially in evaluating retention and precorneal clearance. The developed sparfloxacin in situ gel formulation was nonirritant and showed prolonged retention at the corneal site with prolonged release. Formulation was found suitable for sustained topical drug delivery to eyes for rational drug therapy in case of various ocular diseases.
Future prospective
In situ gel technique is emerging as a practical solution in enhancing the ocular retention for drugs. With the use of mucoadhesive polymers that is, chitosan we can demonstrate the better efficacy of the present formulation in terms of ocular retention and tolerance. This work is preliminary work to show the proof of concept and utility of the novel combination of chitosan. Third generation fluroquinolone that is, sparfloxacin was used as model drug to prove this concept. Various other drugs can be used in this base in situ gel formulation. The future work includes the optimizing this formulation base with other drug moieties and study its effectiveness. Pharmacokinetic study can be performed to further proof the utility of the formulation.
Financial support and sponsorship
Authors are thankful to Council of Scientific and Industrial Research for providing SRF fellowship to Mr. Himanshu Gupta for carrying out this research work.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Maurice DM. Kinetics of topically applied drugs. In: Saettone MS, Bucci P, Padova S, editors. Ophthalmic Drug Delivery: Biopharmaceutical, Technological and Clinical Aspects. Vol. 11. Padova: Fidia Research Series, Liviana Press; 1987. pp. 19–26. [Google Scholar]
- 2.Schoenwald RD. Ocular drug delivery. Pharmacokinetic considerations. Clin Pharmacokinet. 1990;18:255–69. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 3.Middleton DL, Leung SS, Robinson JR. In: Bioadhesive Drug Delivery Systems. Lenaerts V, Gurny R, editors. Boca Raton, FL: CRC Press; 1990. pp. 179–202. [Google Scholar]
- 4.Swarbrick J, Boylan J. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker; 1995. Ocular drug formulation and delivery; pp. 43–75. [Google Scholar]
- 5.Ranade VV, Hollinger MA. Drug Delivery Systems. Boca Raton, FL: CRC Press; 1996. Intranasal and ocular drug delivery; pp. 209–38. [Google Scholar]
- 6.Felt O, Baeyens V. Encyclopedia of Controlled Drug Delivery. II. NY: USA John Wiley and Sons; 1999. Mucosal drug delivery: Ocular; pp. 605–26. [Google Scholar]
- 7.Chiou GC, Watanabe K. Drug delivery to the eyes. In: Ihler GM, editor. Methods of Drug Delivery: International Encyclopedia of Pharmacology and Therapeutics. Section 120. UK: Pergamon Press; 1986. pp. 203–210. [Google Scholar]
- 8.Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14:507–15. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 9.Singh AK, Bhardwaj N, Bhatnagar A. Pharmacoscintigraphy: An unexplored modality in India. Indian J Pharm Sci. 2004;66:18–25. [Google Scholar]
- 10.Davis SS, Hardy JG, Newman SP, Wilding IR. Gamma scintigraphy in the evaluation of pharmaceutical dosage forms. Eur J Nucl Med. 1992;19:971–86. doi: 10.1007/BF00175865. [DOI] [PubMed] [Google Scholar]
- 11. [Last accessed on 2014 Sep 29]. Available from: http://www.toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+1184 .
- 12.Lin HR, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release. 2000;69:379–88. doi: 10.1016/s0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 13.Tavaszi J, Budai P. The use of HET-CAM test in detecting the ocular irritation. Commun Agric Appl Biol Sci. 2007;72:137–41. [PubMed] [Google Scholar]
- 14. [Last accessed on 2014 Sep 12]. Available from: http://www.hetcam.com .
- 15.Velpandian T, Bankoti R, Humayun S, Ravi AK, Kumari SS, Biswas NR. Comparative evaluation of possible ocular photochemical toxicity of fluoroquinolones meant for ocular use in experimental models. Indian J Exp Biol. 2006;44:387–91. [PubMed] [Google Scholar]
- 16.Spielmann H. Ocular irritation. In: Castle JV, Gomez MJ, editors. In Vitro Methods in Pharmaceutical Research. San Diego, CA: Academic Press; 1997. pp. 265–87. [Google Scholar]


