Abstract
Alcohol abuse is a leading cause of liver disease characterized by liver inflammation, fatty liver, alcoholic hepatitis, or liver cirrhosis. Immunomodulatory effects of alcohol on monocytes and macrophages contribute to alcoholic liver disease. Alcohol use, an independent risk factor for progression of hepatitis C virus (HCV) infection–mediated liver disease, impairs host defense and alters cytokine production and monocyte/macrophage activation. We hypothesized that alcohol and HCV have synergistic effects on the phenotype and function of monocytes. Our data show that acute alcohol binge drinking in healthy volunteers results in increased frequency of CD16+ and CD68+ and M2-type (CD206+, dendritic cell [DC]-SIGN+–expressing and IL-10–secreting) circulating CD14+ monocytes. Expression of HCV-induced CD68 and M2 markers (CD206 and DC-SIGN) in normal monocytes was further enhanced in the presence of alcohol. The levels of microRNA (miR)-27a was significantly upregulated in monocytes cultured in the presence of alcohol or alcohol and HCV as compared with HCV alone. The functional role of miR-27a in macrophage polarization was demonstrated by transfecting monocytes with an miR-27a inhibitor that resulted in reduced alcohol- and HCV- mediated monocyte activation (CD14 and CD68 expression), polarization (CD206 and DC-SIGN expression), and IL-10 secretion. Over-expression of miR-27a in monocytes enhanced IL-10 secretion via activation of the ERK signaling pathway. We found that miR-27a promoted ERK phosphorylation by downregulating the expression of ERK inhibitor sprouty2 in monocytes. Thus, we identified that sprouty2 is a target of miR-27a in human monocytes. In summary, our study demonstrates the regulatory role of miR-27a in alcohol-induced monocyte activation and polarization.
Alcohol is the most commonly used and abused recreational substance, which causes liver disease in >10 million people in the United States (1). Low- to moderate-dose alcohol consumption is associated with reduced risk of coronary heart disease (2, 3). However, excessive alcohol consumption is linked with numerous negative effects, including susceptibility to viral infections (3–5). Binge drinking is the most common form of alcohol consumption in young adults and it is also common in chronic alcoholics. Metabolism of alcohol triggers activation of the innate immune cells in the liver, which contributes to the development and pathogenesis of alcoholic liver diseases (ALD) (6, 7).
It is estimated that 130–150 million people worldwide have chronic hepatitis C virus (HCV) infection (World Health Organization; http://www.who.int/mediacentre/factsheets/fs164/en/), including 3.2 million Americans (Centers for Disease Control and Prevention; http://www.cdc.gov/hepatitis/c/cfaq.htm). Of these, ~70% have a history of alcohol abuse (8). Alcohol predisposes HCV-infected patients to liver cirrhosis, liver cancer, and increased mortality (9–11). Cells of the innate immune system, monocytes and macrophages, are activated with alcohol exposure, and increased macrophage numbers have been reported in the liver in ALD (7, 12). Monocyte/macrophage activation occurs in chronic HCV infection, and dysfunctional macrophages contribute to disease progression and liver fibrosis (13, 14). HCV, a single-stranded RNA virus, can be sensed by monocytes via TLR8. A previous study from our group has shown that alcohol exposure modulates monocytic response to TLR8 stimulation (15). However, the mechanism by which alcohol modulates the monocyte response to HCV infection is not known.
Liver-resident macrophages (Kupffer cells) are one of the major innate immune cell populations; however, during injury and infection, bone marrow–derived and circulating monocytes also enter the liver for immune surveillance (16, 17). Monocytes differentiate into macrophages, and in response to various signals macrophages undergo polarization into classically activated (M1 or proinflammatory) or alternatively activated (M2 or anti-inflammatory) phenotypes (18, 19). M1 macrophages can be generated in the presence of IFN-γ and/or LPS and they exhibit potent antimicrobial properties and promote Th1 responses whereas IL-4, IL-13, IL-10, TGF-β, or glucocorticoids stimulate the M2 macrophage phenotype that supports Th2-associated effector functions and resolution of inflammation (19–21). Cell surface markers such as CD86, MHC class II, CD40, and CD16 are highly expressed on M1 macrophages, whereas M2 macrophages predominantly express CD206 (mannose receptor), dendritic cell (DC)-SIGN, and CD163 (scavenger receptor) (22).
Increasing evidence suggests that microRNAs (miRs) play an important role in macrophage polarization and function (23–25). miRs are small noncoding RNA molecules that are specific for multiple target sequences. In immune cells, miRs affect the function of the innate and adaptive immune response through regulation of cellular differentiation and function (25, 26). The aim of the present study was to elucidate the mechanisms by which alcohol modulates monocyte differentiation, macrophage polarization, and function. Our results demonstrate that healthy human monocytes cultured in the presence of HCV are primed by alcohol to differentiate and polarize into M2 macrophages. Alcohol mediates this process by increasing expression of miR-27a, which activates the ERK signaling pathway and IL-10 secretion via targeting the ERK inhibitor, sprouty2.
Materials and Methods
In vivo human studies
For the in vivo study, healthy individuals (4 males and 13 females, age range 23–57 y) who did not have a history of alcohol-use disorder were enrolled. Written informed consent was obtained from the volunteers. The samples were de-identified and the consent files stored in locked cabinets. The Institutional Review Board for the protection of human subjects in research at the University of Massachusetts Medical School approved the study. Alcohol was given at a dose of 2 ml vodka 40% (v/v) ethanol/kg body weight in a total volume of 300 ml orange juice at the Clinical Research Center, University of Massachusetts Medical School. Blood was drawn at baseline (120 ml) and after every 30 min for the first 4 h (6 ml each time) and again 24 h after alcohol consumption (120 ml).
Alcohol concentration assay
The serum samples were analyzed for the determination of alcohol. This was performed in an Analox alcohol analyzer as per the manufacturer's instructions (Analox Instruments, Lunenberg, MA).
Reagents
DMEM and RPMI 1640 cell culture media, antibiotics, and nonessential amino acids were purchased from Life Technologies (Grand Island, NY). CD14+ monocytes were isolated by MACS CD14 microbeads from Miltenyi Biotec (Auburn, CA). Human Abs, including CD16 allophycocyanin, CD16 FITC, CD14 FITC, CD40 FITC, and CD86 FITC, were purchased from eBioscience (San Diego, CA). Abs CD14 allophycocyanin, CD14 PE, CD163 PE, CD11c allophycocyanin, CD68 PE, CD206 allophycocyanin, DC-SIGN FITC, and isotype control Abs were purchased from BD Pharmingen (Franklin Lakes, NJ). Phospho-p44/42-ERK1/2 and anti-mouse IgG PE were obtained from Cell Signaling Technology (Danvers, MA). Human IL-10 Ab and mouse IgG1 isotype control were from R&D Systems (Minneapolis, MN). miR-27a inhibitor, mimic, and scrambled controls were purchased from Ambion Life Technologies (Carlsbad, CA). Lipofectamine RNAiMAX transfection reagent was from Life Technologies. The sprouty2 construct was obtained from OriGene (Rockville, MD), which was transfected by Roche (Indianapolis, IN) X-tremeGENE transfection reagent. ERK inhibitor, U0126, was procured from EMD Millipore (Billerica, MA).
Biological materials
PBMCs were obtained from healthy individuals aged 18–60 y, females and males with no previous alcohol abuse history who consumed <6 drinks/wk and from individuals recruited in the in vivo study. The study was reviewed and approved by the Institutional Review Board for Protection of Human Subjects in Research. Monocytes from human peripheral blood were isolated by MACS CD14 microbeads following the manufacturer's instructions to ~95% purity (data not shown).
Huh7.5 cells were maintained in low-glucose DMEM containing 10% FBS (HyClone, Logan, UT), 10 μg/ml ciprofloxacin, and supplemented with nonessential amino acids. Transfection of JFH1 RNA into Huh7.5 cells and production of infectious virus in cell culture were performed as previously described (27). HCV viral supernatants were collected from Huh7.5 cells highly infected with JFH1 HCVcc (cell culture–derived HCV particles) and determined by flow cytometry (27). The supernatant was passed through 0.22-μm filters and concentrated 20–30 times using an Amicon Ultra-15 100K centrifugal filter unit, and virus stock was preserved at −80°C (Millipore). Uninfected Huh7.5 cell supernatant was collected, concentrated, and used as controls in the experiments with HCV concentrate.
Coculture experiments
Monocytes (5 × 105 cells) were cocultured with either Huh7.5 cells or HCV-infected hepatoma (Huh7.5/JFH1) cells (48 h postinfection) or HCV concentrate (cell-free HCV) and Huh7.5 concentrate (uninfected control) in 12-well plates in a 37°C, 5% CO2 incubator for 7 d. Ethanol at a concentration of 25 mM was maintained in one experimental plate and another plate did not receive any alcohol. Supernatants, cells, and RNA were collected from each experiment at the indicated time points and surface markers, cytokines, and miR expression were studied.
Flow cytometry
Cell surface markers on PBMCs and monocytes were analyzed by flow cytometry as described previously (27). To inhibit nonspecific binding of Abs, PBMCs and monocytes were incubated with FcR blocking reagent (Miltenyi Biotec). The cells were incubated with appropriate Ab or isotype control for 30 min at 4°C. Cells were washed with FACS buffer and acquired on a BD LSR II (BD Biosciences, San Diego, CA).
Data analysis was performed on FlowJo software (Tree Star, Ashland, OR). For PBMCs, the cells were gated on CD14+ monocytes and were further gated on the other monocyte/macrophage markers as described in the figures. For CD14+ monocytes, we gated on the various markers and calculated the mean fluorescence intensity (MFI). The MFI data were plotted as fold difference as compared with the respective untreated control cells.
ELISA
Levels of human TNF-α and MCP-1 in the cell culture supernatant were measured using commercially available ELISA kits from BD Biosciences (Franklin Lakes, CA) following the manufacturer's instructions. Human IL-10 and TGF-β ELISA kits were purchased from eBioscience.
miR analysis
QIAzol lysis reagent (Qiagen, Germantown, MD) was used to lyse the cells. The lysate was incubated on ice for 5 min before miR isolation using a Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA). Reverse transcription (30 min at 16°C, 30 min at 42°C, 5 min at 85°C) was performed in an Eppendorf Mastercycler ep realplex (Eppendorf, Westbury, NY) using 10 ng RNA, TaqMan primers, and an miRNA reverse transcription kit followed by quantitative RT-PCR (qRT-PCR) (10 min at 95°C, 40 cycles of 15 s at 95°C, 1 min at 60°C) in an iCycler (Bio-Rad Laboratories) using TaqMan Universal PCR master mix and human primers for RNU48 as normalizing control, miR-27a, miR-132, miR-146a, and miR-155. Relative expression was calculated by the ΔΔCt method.
miR inhibition and overexpression studies
For miR-27a inhibition studies, monocytes were transfected with anti–miR-27a and anti–miR-negative control 1 (anti–miR control), and for miR-27a overexpression studies, monocytes were transfected with miR-27a mimic and miR control 1 (miR control) using Lipofectamine RNAiMAX transfection reagent (28). Knockdown and overexpression efficiency was determined by the miR-27a levels in transfected cells. Transfected cells were treated with and without alcohol and/or HCV for indicated duration according to experimental requirements before the isolation of RNA (Direct-zol RNA MiniPrep kit) or supernatant collection.
p-ERK detection and ERK inhibition assay
p-ERK detection was based on a previously described protocol (29). Briefly, monocytes stimulated under various conditions as described in Figs. 5 and 6 were fixed with 2% formaldehyde and permeabilized with 90% methanol. Staining was performed with anti–phospho-p44/42 MAPK (ERK1/2) Ab from Cell Signaling Technology (clone E10). PE-conjugated anti-mouse IgG secondary Ab was used and the cells were acquired and analyzed on a flow cytometer. ERK inhibitor U0126 was used at various doses to inhibit p-ERK expression as described in Results.
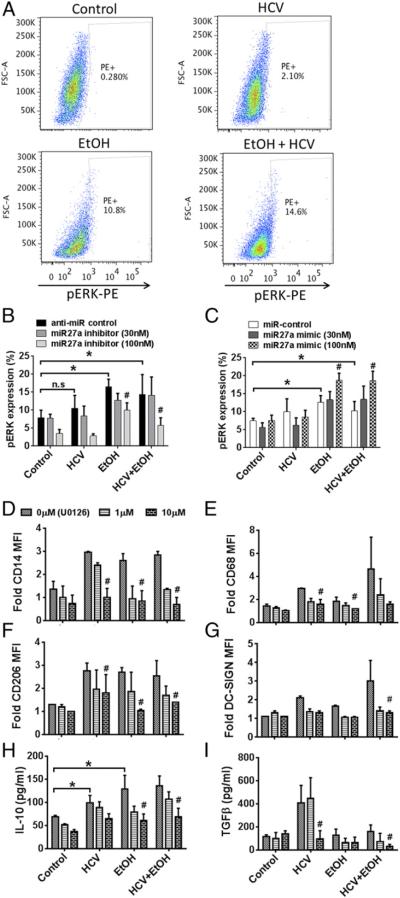
FIGURE 5.
miR-27a regulates IL-10 secretion via ERK activation. (A) Healthy monocytes were stimulated with 25 mM alcohol and/or HCV concentrate. Phosphorylated ERK levels were determined by flow cytometry. Representative dot plot for each treatment condition is shown. (B and C) Healthy monocytes were transfected with anti–miR control or miR-27a inhibitor (B) or miR control or miR-27a mimic (C), stimulated with 25 mM alcohol and/or HCV concentrate for 7 d as indicated. Phosphorylated ERK levels were determined by flow cytometry, and the data are represented as means ± SEM (n = 3). *p < 0.05. #p < 0.05 as compared with anti–miR control or miR control. (D–I) Monocytes were pretreated with various doses of U0126 and then stimulated with alcohol and HCV as indicated. (D–G) Cell surface expression of CD14, CD68, CD206, and DC-SIGN were determined on the monocytes by flow cytometry. The bar graphs represent the MFI of the indicated proteins. (H and I) Cell culture supernatants were tested for IL-10 and TGF-β secretion by ELISA. The data are represented as means ± SEM (n = 4 experiments). *p < 0.05. #p < 0.05 compared with the respective 0 μM U0126 control. EtOH, ethanol.
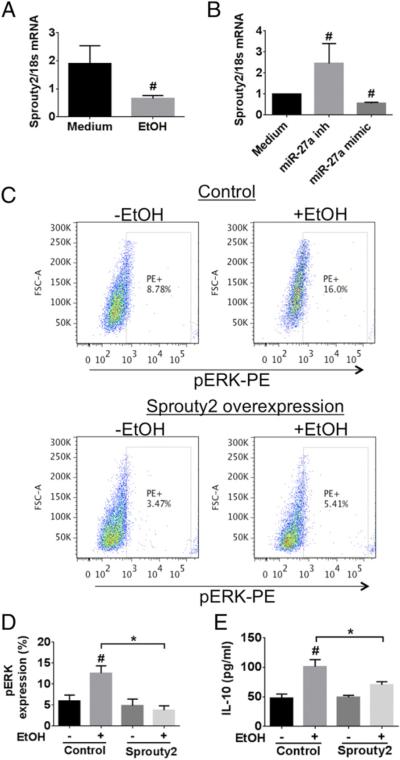
FIGURE 6.
Sprouty2 levels are modulated by miR-27a in the monocytes treated with ethanol. (A) Monocytes were treated with 25 mM EtOH for 24 h and the mRNA level of sprouty2 was determined. (B) Monocytes transfected with or without miR-27a inhibitor or miR-27a mimic were evaluated for the mRNA levels of sprouty2. (C) Sprouty2 overexpressing monocytes were stimulated with 25 mM EtOH for 24 h. We determined the levels of p-ERK by flow cytometry. Representative dot plots for each treatment condition are shown. The percentages of PE+ cells are the cells expressing activated p-ERK. (D and E) Healthy monocytes were transfected with sprouty2 overexpression construct and were stimulated with 25 mM EtOH for 24 h. (D) Levels of p-ERK were determined by flow cytometry. (E) Levels of IL-10 cytokine were determined in the culture supernatant by ELISA. The data are represented as means ± SEM of three independent experiments. *p < 0.05. #p < 0.05 compared with medium or control. EtOH, ethanol.
Sprouty2 overexpression
For sprouty2 overexpression experiments, monocytes were transfected with 0.1 μg PCMV-SPRY2 construct (OriGene) by a Roche X-treme transfection kit. Sprouty2 overexpression was confirmed by gene expression analysis (Supplemental Fig. 4).
Statistical analysis
All values (expressed as mean ± SEM) were obtained from three or more independent experiments. Comparison between groups was made with the Student t test or ANOVA test. A p value <0.05 was considered statistically significant. GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for statistical analysis.
Results
In vivo acute alcohol binge induces monocyte activation in healthy subjects
To investigate whether alcohol consumption induces monocyte activation in vivo, we evaluated the phenotype and function of circulating monocytes from normal, nonalcoholic individuals before and after acute alcohol binge drinking. Whole-blood samples were obtained before and after every 30 min for the first 4 h and again 24 h after consumption of alcohol equivalent to four to five standard drinks (0.85 g/kg body weight). Blood alcohol levels reached maximal levels of 10 mg/dl by 1 h after alcohol consumption (Supplemental Fig. 1). The average blood alcohol level at the time of 4 h after alcohol sampling was <40 mg/dl (Supplemental Fig. 1) and undetectable by 24 h (data not shown).
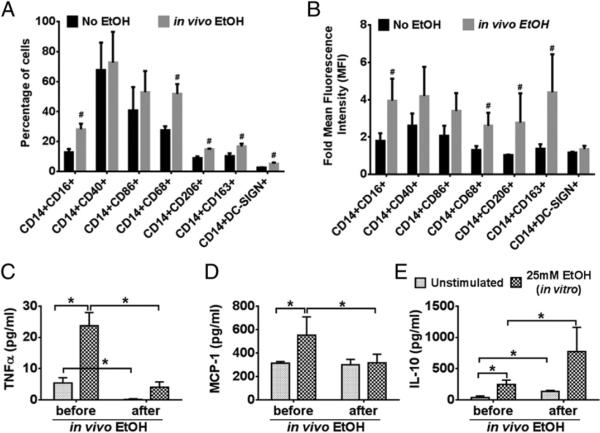
PBMCs were immunophenotyped for monocyte markers and analyzed by flow cytometry. Compared to baseline we observed a significant increase in the percentage of circulating CD14+CD16+ monocytes in healthy individuals after alcohol consumption, and there was no change in the frequency of monocytes expressing CD40 and CD86 (Fig. 1A). Alcohol consumption also resulted in a significant increase in the frequency of monocytes expressing the macrophage marker CD68 (Fig. 1A, Supplemental Fig. 2). Importantly, acute alcohol consumption led to an increase in the percentage of monocytes expressing the well-known M2 markers, CD206, CD163, and DC-SIGN (Fig. 1A, Supplemental Fig. 2). We also observed an increase in the expression levels (MFI) of CD16, CD68, CD206, and CD163 on the CD14+ monocytes after acute alcohol consumption (Fig. 1B). These results showed that activation as well as phenotypic modulation of monocytes occurred after acute alcohol binge drinking.
FIGURE 1.
Acute alcohol intake leads to monocyte activation in vivo. Normal, nonalcoholic individuals received acute alcohol binge (2 ml vodka/kg body weight). Blood samples were taken before and 24 h after alcohol intake. PBMCs were isolated and immunophenotyped by flow cytometry. (A and B) Frequency and MFI of CD14+ cells expressing various markers as mentioned in the figure are depicted in the bar graphs. The data are represented as means ± SEM (n = 10 individuals). #p < 0.05 compared with the “No EtOH” group. (C–E) Monocytes were isolated from the PBMCs mentioned above and were stimulated in vitro with 25 mM EtOH for 24 h. Levels of TNF-α, MCP-1, and IL-10 were determined by ELISA. The data are represented as means ± SEM (n = 10 individuals). *p < 0.05. EtOH, ethanol.
To further evaluate the effects of alcohol, monocytes were isolated from the PBMCs before and after alcohol binge and stimulated ex vivo with 25 mM ethanol. Secreted TNF-α, MCP-1, and IL-10 levels were assessed in the culture supernatant. A significant decrease was seen in TNF-α induction in response to ex vivo ethanol stimulation in monocytes obtained on day 2 after alcohol binge (Fig. 1C). However, we did not observe such an effect on MCP-1 levels (Fig. 1D). Acute alcohol binge resulted in a significant increase in IL-10 secretion by monocytes, which were further increased by ex vivo ethanol stimulation (Fig. 1E). These results demonstrate that acute alcohol consumption leads to changes in the monocyte phenotype that attenuate proinflammatory cytokines and enhance anti-inflammatory cytokine production.
Acute alcohol potentiates the effect of HCV on monocytes
We have previously shown that both acute and chronic alcohol interferes with type I IFN production by monocytes in response to viral and bacterial pathogen-derived signals (15). Monocyte activation has been observed during chronic HCV infection (14, 30). In fact, increased IL-10 and lowered IL-12 secretion by monocytes and macrophages during HCV infection have been shown to have inhibitory effects on antiviral T cell response (31, 32). In the present study, we tested the hypothesis that acute alcohol can synergize with HCV and modulate monocyte phenotype and function. In HCV-infected individuals monocytes/macrophages are exposed to circulating HCV viral particles derived from hepatocytes, the cell type that hosts viral replication (30).
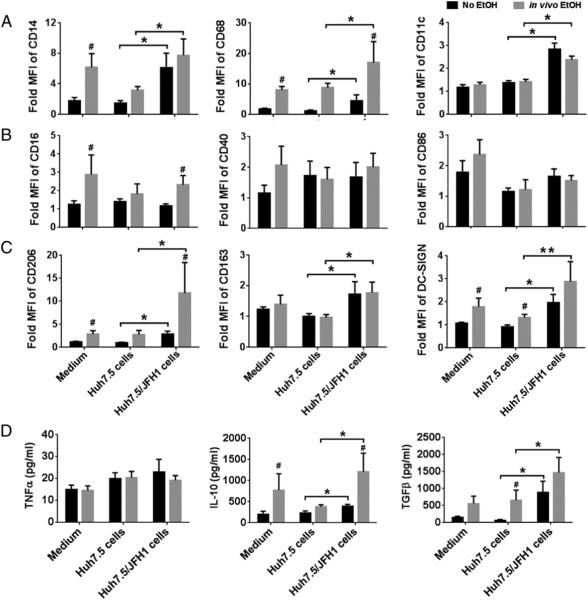
To mimic the interaction between peripheral blood monocytes and HCV-infected hepatocytes, we obtained PBMCs from healthy control individuals before and 24 h after alcohol consumption and cocultured them with Huh7.5 cells or Huh7.5 cells infected with JFH1 (HCV) for 7 d. We observed that monocytes obtained 24 h after alcohol had a high expression of CD14, CD68, CD16, CD206, and DC-SIGN compared with baseline (Fig. 2A–C). We also observed that monocytes cultured in the presence of HCV-infected hepatocytes had significantly increased expression of macrophage markers, CD14, CD68, and CD11c (Fig. 2A) and M2 markers CD206, CD163, and DC-SIGN (Fig. 2C) compared with monocytes cocultured with HCV uninfected hepatoma cells (Huh7.5) or unstimulated cells. Alcohol and HCV had synergistic effects on the upregulation of macrophage markers, CD68 and M2 markers, CD206, and DC-SIGN on the monocytes (Fig. 2A–C). The levels of costimulatory markers CD40 and CD86 on monocytes were not modulated by either alcohol or HCV (Fig. 2B).
FIGURE 2.
Acute alcohol enhances HCV-induced phenotypic changes and cytokine secretion. Monocytes isolated from individuals before and 24 h after acute alcohol binge were cocultured in the presence of Huh7.5/JFH1 cells or uninfected Huh7.5 cells for 7 d. Cell surface expression of (A) CD14, CD68, and CD11c, (B) M1 markers CD16, CD40, and CD68 and (C) M2 markers CD206, CD163, and DC-SIGN were determined by flow cytometry. (D) Cytokine levels of TNF-α, IL-10, and TGF-β were measured from the culture supernatants of the cocultures. The data are represented as means ± SEM (n = 5–7 individuals). *p < 0.05, **p < 0.01. #p < 0.05 compared with “No EtOH” group. EtOH, ethanol.
Cytokine production is another important indicator of monocyte activation, and thus we determined the cytokine secretion over a period of 7 d following alcohol and HCV stimulation. Levels of anti-inflammatory cytokines, IL-10 and TGF-β, were increased in monocytes after alcohol treatment (Fig. 2D). Alcohol and HCV had a synergistic effect on increasing monocyte secretion of IL-10 but not TNF-α. These results suggest that alcohol-exposed monocytes are more susceptible to changes in the phenotype and function mediated by HCV.
Expression profile of miRs in alcohol and HCV-exposed monocytes
Recent studies have identified that alcohol or HCV infection alters monocyte/macrophage miR expression (28, 33). miRs also regulate the process of monocyte differentiation and macrophage polarization (23, 34). Thus, we hypothesized that alcohol may act via miRs to change the monocyte phenotype. Monocytes were differentiated in the presence of M-CSF and polarized to M1 or M2 phenotype by treatment with IFN-γ and LPS or IL-4, respectively (34). We found increased expression of miR-155, miR-146a, and miR-132 in M1 polarized macrophages (Supplemental Fig. 3). miR-132 and miR-27a levels were upregulated in M2 macrophages (Supplemental Fig. 3). Monocytes obtained from individuals after binge drinking had significantly increased expression of miR-27a, miR-146a, and miR-132 but no change in miR-155 (Fig. 3).
FIGURE 3.
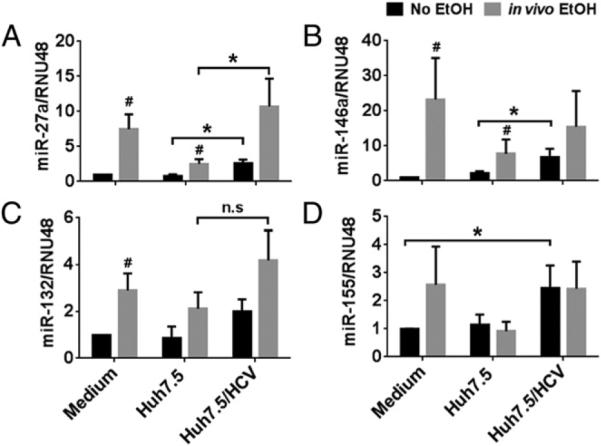
Expression levels of miRs involved in monocyte differentiation and polarization changes with alcohol intake. (A–D) Monocytes isolated from individuals before and 24 h after acute alcohol binge were cocultured in the presence of HCV concentrate or control concentrate for 7 d. The cells were harvested and the expression of miR-27a, miR-146a, miR-132, and miR-155 were assayed by qRT-PCR, and data were normalized to RNU48 control. The fold increase in the expression of these miRs versus nonstimulated cells is shown. The data are represented as means ± SEM of at least four independent experiments. *p < 0.05. #p < 0.05 compared with “No EtOH” group. EtOH, ethanol.
Next, we investigated the effect of cell-free HCV on miR expression in normal and alcohol-exposed monocytes. Coculture of monocytes with Huh7.5/JFH-1 cells or with cell-free HCV viral particles had similar effects on monocyte phenotype and function (B. Saha, K. Kodys, and G. Szabo, submitted for publication); thus, we are showing the data only for the cell-free HCV. We observed an additive effect of in vivo alcohol and HCV on the monocytes selective to miR-27a upregulation, as we found no increase in miR-155, miR-146a, and miR-132 (Fig. 3). These results suggest that the effect of alcohol and HCVon the monocyte phenotype and function might be mediated by miR-27a.
Functional role of miR-27a in alcohol and HCV-mediated monocyte differentiation and polarization
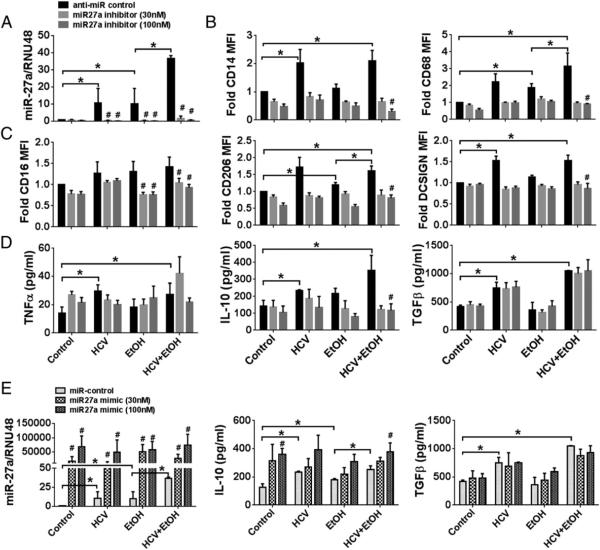
Induction of miR-27a by alcohol and HCV was further confirmed in vitro (Fig. 4A). To evaluate the functional role of miR-27a on monocyte differentiation and polarization by alcohol in the presence of HCV, we overexpressed an miR-27a inhibitor in monocytes and in vitro exposed them to alcohol and HCV for 7 d. As expected, the alcohol- and HCV-induced miR-27a increase was prevented in the presence of miR-27a inhibitor (Fig. 4A). The increase in HCV and alcohol-induced CD14 and CD68 was prevented by the presence of the miR-27a inhibitor but not the anti–miR control (Fig. 4B). We also observed reduced expression of CD206 and DC-SIGN surface markers on alcohol plus HCV-exposed monocytes in the presence of the miR27a inhibitor compared with anti–miR control (Fig. 4C).
FIGURE 4.
miR-27a targets alcohol- and HCV-mediated monocyte activation and IL-10 secretion. (A–E) Healthy monocytes were transfected with anti– miR control or miR-27a inhibitor (A–D) or miR control or miR-27a mimic (E), stimulated with 25 mM alcohol and/or HCV concentrate for 7 d (A–E) as indicated. (A) miR-27a levels were determined by qRT-PCR. (B and C) Cell surface expression of CD14, CD68, CD16, CD206, and DC-SIGN were determined on the monocytes. (D) Culture medium was collected and supernatants were analyzed for TNF-α, IL-10, and TGF-β production by ELISA. (E) miR-27a expression was assayed by qRT-PCR and normalized to RNU48 control after transfection with miR control or miR-27a mimic. Data from three experiments (means ± SEM) are shown. *p < 0.05. #p < 0.05 as compared with anti–miR control or miR control. EtOH, ethanol.
Transfection of miR-27a inhibitor led to decreased secretion of IL-10 but no change in the levels of TNF-α or TGF-β (Fig. 4D). A significant decrease was found in IL-10 levels in monocytes that were treated with alcohol or alcohol plus HCV in the presence of the miR-27a inhibitor as compared with control inhibitor (Fig. 4D). Conversely, overexpression of an miR-27a mimic resulted in increased IL-10 levels in monocytes. miR-27a mimic further increased alcohol- and HCV-induced IL-10 levels (Fig. 4E). The TGF-β levels were not modulated with miR-27a mimic overexpression (Fig. 4E). These results suggest that miR-27a might exert its effects on monocytes via regulating the expression of IL-10. Furthermore, our results indicate that some of the effects of the alcohol on monocytes are mediated via miR-27a induction.
miR-27a regulates alcohol-induced M2 polarization via the ERK signaling pathway
The MAPK/ERK pathway critically regulates a variety of physiological processes, such as cell growth, differentiation, survival, and many others. In immune cells, such as monocytes/macrophages, plasmacytoid DCs, and myeloid DCs, IL-10 expression following TLR stimulation is dependent on ERK activation (35). Also, over-expression of miR-27a promotes the expression of IL-10 in human monocyte-derived DCs (36). Thus, we investigated the role of miR-27a in ERK activation in the monocytes exposed to alcohol and HCV.
We observed increased p-ERK levels in the alcohol- and alcohol plus HCV–treated monocytes (Fig. 5A–C). The increase in p-ERK was dependent on miR-27a levels, as the miR-27a inhibitor reduced and miR-27a mimic increased p-ERK levels (Fig. 5B, 5C). HCV had no effect on alcohol-induced p-ERK levels. Furthermore, to demonstrate the role of ERK activation in IL-10 secretion, monocytes were pretreated with U0126, a p-ERK inhibitor. A dose-dependent reduction in the expression of CD14, CD68, CD206, and DC-SIGN was observed with U0126 (Fig. 5D–G). Furthermore, we measured the levels of IL-10 and TGF-β in a similar experimental setting and again observed a dose-dependent reduction in alcohol-induced IL-10 levels in monocytes pretreated with U0126 (Fig. 5H). ERK inhibitor lowered TGF-β levels in monocytes cultured with HCV alone but not with alcohol (Fig. 5I). These results suggested a role of p-ERK in alcohol-mediated IL-10 induction.
Sprouty2, the connecting link between miR-27a and ERK signaling
Sprouty2 is a validated target of miR-27a. In lung cancer and angiogenesis it negatively regulates the ERK/MAPK pathway (37, 38). Because our results demonstrated that increased miR-27a leads to increased ERK activation and IL-10 secretion, we investigated the role of sprouty2 in alcohol-induced IL-10 production. As HCV did not have any effect on p-ERK levels, we focused our study on the role of sprouty2 in monocytes exposed to alcohol.
We found that sprouty2 gene expression was decreased in monocytes treated with ethanol (Fig. 6A). Second, we observed a significant increase in the gene expression of sprouty2 in the presence of an miR-27a inhibitor (Fig. 6B). Conversely, the miR-27a mimic had an opposite effect on monocytes and lowered sprouty2 expression levels (Fig. 6B). These results demonstrate that sprouty2 gene expression was dependent on miR-27a levels in monocytes.
Finally, to investigate the functional role of sprouty2 in alcohol-mediated ERK signaling and IL-10 secretion, we performed sprouty2 overexpression studies in healthy monocytes (Supplemental Fig. 4). We observed that monocytes overexpressing sprouty2 showed a significant reduction in p-ERK expression with alcohol treatment (Fig. 6C, 6D). Furthermore, IL-10 secretion by monocytes exposed to ethanol was also significantly reduced in the presence of sprouty2 overexpression (Fig. 6E). Taken together, these results demonstrate that the increased miR-27a levels in monocytes treated with alcohol lower the sprouty2 levels, which in turn relieves the inhibitory effect on ERK and subsequently leads to increased IL-10 secretion, and in the presence of increased sprouty2 this effect is reversed (Fig. 7).
FIGURE 7.
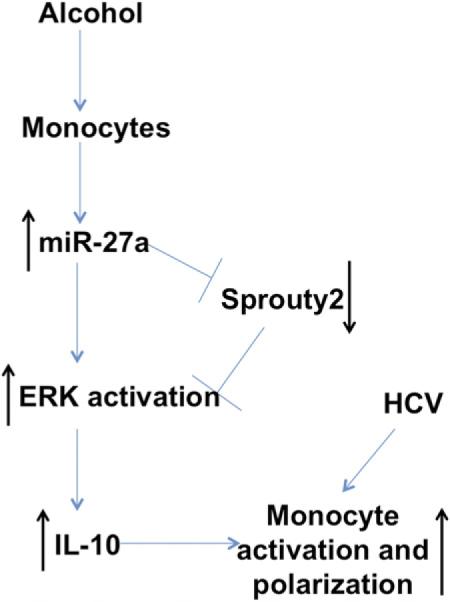
Alcohol mediates monocyte activation and polarization via miR-27a. Monocytes exposed to alcohol lead to increase in miR-27a levels, which target sprouty2, an inhibitor of ERK activation. Decreased sprouty2 levels relieve the inhibitory effect on ERK activation. This leads to increased IL-10 secretion by monocytes. Alcohol synergizes with HCV to mediate monocyte activation and polarization.
Discussion
In this study we demonstrate that alcohol intake leads to monocyte activation and polarization in vivo. We show that alcohol has a synergistic effect on HCV-mediated monocyte differentiation and macrophage polarization. We demonstrate a novel role of miR-27a in mediating monocyte differentiation and M2 polarization. Our observations suggest that miR-27a regulates ERK-mediated IL-10 secretion by monocytes exposed to ethanol (Fig. 7). Alcohol is a known immunomodulator and alcoholics are highly susceptible to bacterial and viral infections (28, 39). A previous study from our group has also shown that the monocytes exposed to alcohol have impaired antiviral and antibacterial immune responses (28). Thus, the M2 polarization in the presence of alcohol observed in HCV-exposed monocytes is one of the mechanisms that impairs antiviral response during infections (Fig. 7).
Monocytes and macrophages, which are important players in the innate immune system, are highly plastic and are heterogeneous in their phenotype and function. Depending on the environmental cues, monocytes migrate to the tissues and differentiate into macrophages and become polarized. This phenomenon has been reported during liver injury and fibrosis (40). Circulating monocytes are the precursors of the inflammatory macrophages and further polarize into repair macrophages during sterile injury (41). The observation that in vivo alcohol has an effect on the phenotype and function of the circulating monocytes suggests not only that alcohol affects intrahepatic cells but also affects immune cells in the periphery. Studies from French and colleagues (42) have demonstrated the presence of M1 and M2 macrophages in the liver of alcoholic hepatitis patients. They have shown the presence of CD206+ and CD163+ M2 macrophages in the liver of alcoholic hepatitis patients by immunohistochemisrty, which suggests that chronic consumption of alcohol does lead to increased M2-type macrophages in the liver (42). Our results demonstrate that the addition of alcohol to HCV-exposed cells further alters the phenotype and functions of normal monocytes to an M2-polarized, profibrogenic macrophage, demonstrating the role of alcohol in impairing antiviral immune response.
The role of miRs in the process of hematopoiesis, differentiation, survival, and function of immune cells, including monocytes and macrophages, has been demonstrated previously (26, 43). miR-27a is upregulated in a differentiated THP-1 monocytic cell line during Salmonella infection (26). Transfection of miR-29b, miR-125a-5p, or miR-155 mimics in THP-1 cells altered macrophage polarization to M1 macrophages (23). Experiments with human macrophages have identified unique miRs associated with polarized macrophage phenotypes such as miR-155 in M1 macrophages and miR-27a and miR-132 expression in M2 macrophages (23). Thus it is evident that miRs regulate macrophage polarization by altering gene expression under polarizing environmental conditions. In our study we observed that increased miR-27a expression correlated with M2 macrophage phenotype in the alcohol- and HCV-exposed monocytes.
miR-27a is a member of the miR-23~27-24 cluster and is highly expressed in endothelial cells along with miR-27b (44). miR-27a enhances differentiation of myeloblasts into granulocytes by downregulating Runx1 and cell proliferation in several cancers (37, 45). miR-27a targets multiple intracellular signaling networks (37). miR-27a has been shown to inhibit DC-mediated differentiation of Th1 and Th17 cells and promote the DC-mediated accumulation of Tr1 (CD4+IL-10+), regulatory T cells, and increased secretion of IL-10 (36). A recent study has reported that miR-27a regulates the inflammatory response of macrophages by targeting IL-10 and decreasing the levels of IL-10 (46). Results of our study revealed that the effect of miR-27a on IL-10 levels in monocytes is not direct, rather indirect via increasing ERK signaling (Fig. 5). SEMA6A and sprouty2 are both targets of miR-27 in endothelial cells (37, 38, 45). Our results showed that alcohol-treated monocytes increased miR-27a expression, which in turn lowered sprouty2 levels. Transfection experiments with miR-27a inhibitor and mimic also demonstrated that sprouty2 is its target in alcohol-exposed monocytes.
MAPKs such as ERK regulate numerous cellular events associated with the inflammatory response, cellular proliferation, and cell survival (45). The production of IL-10 by macrophages and DCs is mediated by activation of ERK. We and others have found ERK activation in monocytes exposed to alcohol, and the role of ERK activation in alcohol-exposed monocytes is sensitization to other TLR ligands (47, 48). It has been demonstrated that miR-27a inhibited JNK and p38 phosphorylation by targeting MAPK2K4, MAP2K7, and p38 MAPK, whereas ERK activity was not suppressed (45). miR-27a expression has been shown to promote the production of IL-10, which in turn promotes the accumulation of CD4+Foxp3+ regulatory T cells in tumor sites infused with miR-27a transfected DCs (45). In our study we observed that miR-27a indirectly increased ERK activation by targeting sprouty2, which is an inhibitor of ERK1/2. This led to increased levels of IL-10 secretion by monocytes treated with alcohol. We did not observe a change in the ERK levels in monocytes exposed to HCV. However, we observed a decrease in the levels of HCV-induced monocyte/macrophage markers in the presence of ERK inhibitor. This may be attributed to the decreased levels of HCV-induced TGF-β in the presence of ERK inhibitor (Fig. 5I).
The anti-inflammatory cytokine IL-10 has a central role in limiting the immune response to inflammatory and autoimmune pathologies (35). IL-10 acts as a developmental switch guiding monocyte differentiation to macrophages during murine peritoneal infection (49). IL-10 prevents the differentiation of monocytes to DCs but promotes their maturation into macrophages and polarizes to alternatively activated macrophages (50, 51). Thus, the increased IL-10 secreted by monocytes in the presence of alcohol can act as a differentiating factor and generate M2-polarized macrophages. In a situation where alcohol and HCV are both present, we found a synergistic action on altering monocyte phenotype and cytokine secretion pattern. In summary, our study identified the important role of miR-27a in the immunomodulatory effects of alcohol on monocytes.
Further research is required to understand the role of other miRs in alcohol-mediated effects on the monocyte/macrophage regulation. One of the limitations of the present study is that we were unable to assess the role of miR-27a in the liver of the alcoholic patients. However, it would be interesting to study the role of miR-27a in a chronic alcohol model of mice to further decipher the role of this miR in regulating intrahepatic monocyte/macrophage phenotype and cell function, which would lead to a broader and deeper understanding of the role of miR-27a in alcoholic liver diseases.
Supplementary Material
Acknowledgments
We thank Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) and Dr. Charles M. Rice (Rockefeller University, New York, NY) for providing the infectious JFH-1 molecular clone and Huh7.5 cells. We thank staff from Clinical Research Center, including Michelle Kelley and Karen Gallagher-Dorval, and Donna Giansiracusa and Sharon Balcom from the GI research office for assistance with the clinical studies. We greatly appreciate the participation of volunteers for this study.
This work was supported by National Institutes of Health Grants R01 AA011576 (to G.S.) and R01 AA011576S to (J.C.B.).
Abbreviations used in this article
- ALD
alcoholic liver disease
- DC
dendritic cell
- HCV
hepatitis C virus
- MFI
mean fluorescence intensity
- miR
microRNA
- qRTPCR
quantitative RT-PCR
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Yoon Y, Yi HY. Liver cirrhosis mortality in the United States, 1970– 2009. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2012. Surveillance Report no. 93. Available at: http://pubs.niaaa.nih.gov/publications/Surveillance93/Cirr09.htm. [PMC free article] [PubMed] [Google Scholar]
- 2.O'Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ. Alcohol and cardiovascular health: the dose makes the poison ... or the remedy. Mayo Clin. Proc. 2014;89:382–393. doi: 10.1016/j.mayocp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Mukamal KJ, Rimm EB. Alcohol consumption: risks and benefits. Curr. Atheroscler. Rep. 2008;10:536–543. doi: 10.1007/s11883-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch EL, Brown HG, Gamelli RL, Kovacs EJ. Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. J. Burn Care Res. 2008;29:323–330. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, Wands JR, Eken A, Osna NA, Weinman SA, Machida K, Joe Wang H. Alcohol and hepatitis C virus—interactions in immune dysfunctions and liver damage. Alcohol. Clin. Exp. Res. 2010;34:1675–1686. doi: 10.1111/j.1530-0277.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig. Dis. 2012;30(Suppl 1):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 8.Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C infection and alcohol use: a dangerous mix for the liver and antiviral immunity. Alcohol. Clin. Exp. Res. 2006;30:709–719. doi: 10.1111/j.1530-0277.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 9.Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, Dhillon AP, Norkrans G, Wejstål R. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J. Viral Hepat. 2002;9:235–241. doi: 10.1046/j.1365-2893.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 10.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin. Liver Dis. 2004;24:305–315. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- 11.Zakhari S. Bermuda Triangle for the liver: alcohol, obesity, and viral hepatitis. J. Gastroenterol. Hepatol. 2013;28(Suppl. 1):18–25. doi: 10.1111/jgh.12207. [DOI] [PubMed] [Google Scholar]
- 12.Maraslioglu M, Oppermann E, Blattner C, Weber R, Henrich D, Jobin C, Schleucher E, Marzi I, Lehnert M. Chronic ethanol feeding modulates inflammatory mediators, activation of nuclear factor-κB, and responsiveness to endotoxin in murine Kupffer cells and circulating leukocytes. Mediators Inflamm. 2014;2014:808695. doi: 10.1155/2014/808695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo G, Dolganiuc A. Hepatitis C and innate immunity: recent advances. Clin. Liver Dis. 2008;12:675–692, x (x.). doi: 10.1016/j.cld.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of Toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang M, Bala S, Kodys K, Catalano D, Szabo G. Inhibition of TLR8- and TLR4-induced type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes. BMC Immunol. 2011;12:55. doi: 10.1186/1471-2172-12-55. doi:10.1186/1471-2172-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez FO, Gordon S. Tmhe M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc. Natl. Acad. Sci. USA. 2013;110:11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey-Giraud F, Hafner M, Ries CH. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS ONE. 2012;7:e42656. doi: 10.1371/journal.pone.0042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 2012;287:21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Gazzar M, McCall CE. MicroRNAs regulatory networks in myeloid lineage development and differentiation: regulators of the regulators. Immunol. Cell Biol. 2012;90:587–593. doi: 10.1038/icb.2011.74. [DOI] [PubMed] [Google Scholar]
- 25.O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 26.Sharbati S, Sharbati J, Hoeke L, Bohmer M, Einspanier R. Quantification and accurate normalisation of small RNAs through new custom RT-qPCR arrays demonstrates Salmonella-induced microRNAs in human monocytes. BMC Genomics. 2012;13:23. doi: 10.1186/1471-2164-13-23. doi:10.1186/1471-2164-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Kodys K, Li K, Szabo G. Human type 2 myeloid dendritic cells produce interferon-λ and amplify interferon-α in response to hepatitis C virus infection. Gastroenterology. 2013;144:414–425. e7. doi: 10.1053/j.gastro.2012.10.034. doi:10.1053/j.gastro.2012.10.034. Epub 2012 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu YL, Shan L, Huang H, Haupt C, Bessell C, Canaday DH, Zhang H, Ho YC, Powell JD, Oelke M, et al. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J. Clin. Invest. 2014;124:198–208. doi: 10.1172/JCI70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heydtmann M. Macrophages in hepatitis B and hepatitis C virus infections. J. Virol. 2009;83:2796–2802. doi: 10.1128/JVI.00996-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Jia ZS, Moorman JP, Yao ZQ. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS ONE. 2011;6:e19664. doi: 10.1371/journal.pone.0019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bala S, Tilahun Y, Taha O, Alao H, Kodys K, Catalano D, Szabo G. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J. Transl. Med. 2012;10:151. doi: 10.1186/1479-5876-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int. J. Mol. Med. 2013;31:797–802. doi: 10.3892/ijmm.2013.1260. [DOI] [PubMed] [Google Scholar]
- 35.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 36.Min S, Li L, Zhang M, Zhang Y, Liang X, Xie Y, He Q, Li Y, Sun J, Liu Q, et al. TGF-β-associated miR-27a inhibits dendritic cell-mediated differentiation of Th1 and Th17 cells by TAB3, p38 MAPK, MAP2K4 and MAP2K7. Genes Immun. 2012;13:621–631. doi: 10.1038/gene.2012.45. [DOI] [PubMed] [Google Scholar]
- 37.Acunzo M, Romano G, Palmieri D, Laganá A, Garofalo M, Balatti V, Drusco A, Chiariello M, Nana-Sinkam P, Croce CM. Cross-talk between MET and EGFR in non-small cell lung cancer involves miR-27a and Sprouty2. Proc. Natl. Acad. Sci. USA. 2013;110:8573–8578. doi: 10.1073/pnas.1302107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc. Natl. Acad. Sci. USA. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin. Microbiol. Rev. 2003;16:209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL, Jr., Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS ONE. 2014;9:e86660. doi: 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, French B, Morgan T, French SW. The liver is populated by a broad spectrum of markers for macrophages. In alcoholic hepatitis the macrophages are M1 and M2. Exp. Mol. Pathol. 2014;96:118–125. doi: 10.1016/j.yexmp.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Undi RB, Kandi R, Gutti RK. MicroRNAs as haematopoiesis regulators. Adv. Hematol. 2013;2013:695754. doi: 10.1155/2013/695754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbich C, Kaluza D, Frömel T, Knau A, Bennewitz K, Boon RA, Bonauer A, Doebele C, Boeckel JN, Hergenreider E, et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood. 2012;119:1607–1616. doi: 10.1182/blood-2011-08-373886. [DOI] [PubMed] [Google Scholar]
- 45.Feng J, Iwama A, Satake M, Kohu K. MicroRNA-27 enhances differentiation of myeloblasts into granulocytes by post-transcriptionally downregulating Runx1. Br. J. Haematol. 2009;145:412–423. doi: 10.1111/j.1365-2141.2009.07632.x. [DOI] [PubMed] [Google Scholar]
- 46.Xie N, Cui H, Banerjee S, Tan Z, Salomao R, Fu M, Abraham E, Thannickal VJ, Liu G. miR-27a regulates inflammatory response of macrophages by targeting IL-10. J. Immunol. 2014;193:327–334. doi: 10.4049/jimmunol.1400203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen HH, Tran BT, Muller W, Jack RS. IL-10 acts as a developmental switch guiding monocyte differentiation to macrophages during a murine peritoneal infection. J. Immunol. 2012;189:3112–3120. doi: 10.4049/jimmunol.1200360. [DOI] [PubMed] [Google Scholar]
- 50.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 1998;28:359–369. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Prasse A, Germann M, Pechkovsky DV, Markert A, Verres T, Stahl M, Melchers I, Luttmann W, Müller-Quernheim J, Zissel G. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J. Allergy Clin. Immunol. 2007;119:464–471. doi: 10.1016/j.jaci.2006.09.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.