Chart 1.
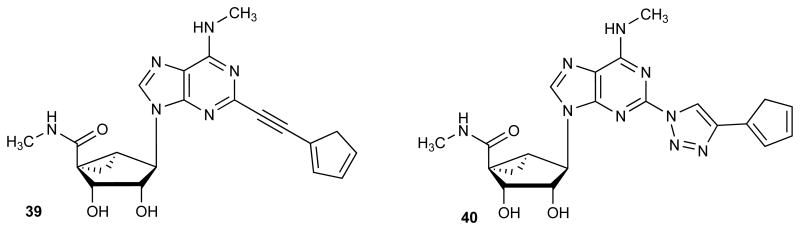
A. Structures of recently reported highly selective A3AR agonists (1, 2) and the structural options considered in the present study (3). R is a small alkyl, cycloalkyl or substituted arylalkyl group; Ar refers to substituted phenyl or heterocyclic groups or ferrocene (See compound 4, Table 2). B. Hypothetical transient breakdown products 39 and 40 from acid treatment of the ferrocene derivatives 4 and 20, respectively. Only 39 was detected by mass upon acidification of 4; compound 20 was stable at pH 1.6.