Figure 1.
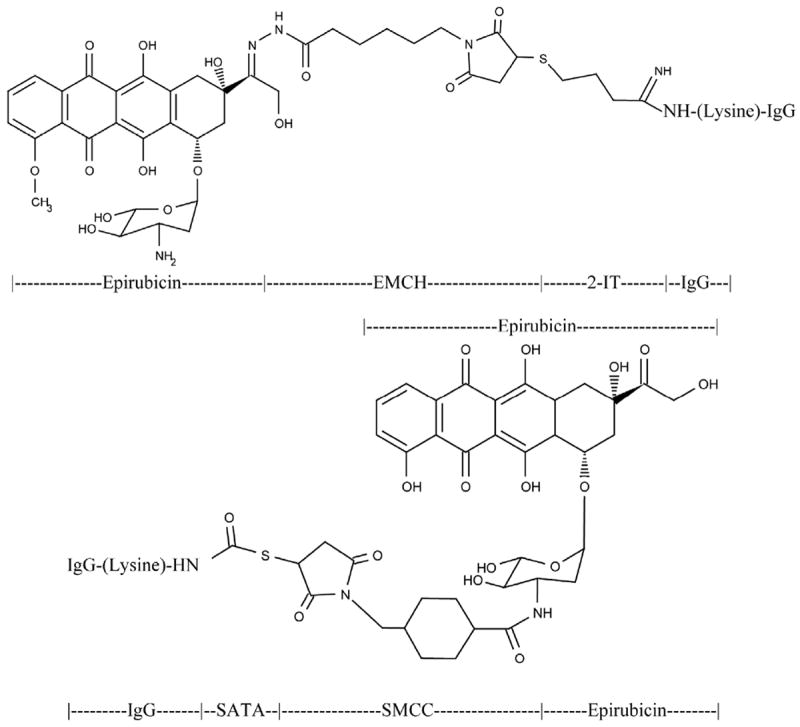
Top Panel-Covalent epirubicin-(C13-imino)-[anti-HER2/neu] immunochemotherapeutic created using a 2-phase synthesis scheme. Phase-I: a maleimodocaproyl-hydrazone is created at the C13-keto group of the anthracycline through the carbonyl-reactive hydrazide group of N-ε-maleimidocaproic acid hydrazide (EMCH). Phase-II: the sulfhydryl-reactive maleimide group of the epirubicin-(C13-imino) maleimodocaproyl-hydrazone intermediate is covalently linked at/to thiolated lysine α-amine groups residing within anti-HER2/neu monoclonal immunoglobulin fractions. Bottom Panel-Covalent epirubicin-(C3-amide)-[anti-HER2/neu] immunochemotherapeutic created using as 2-phase synthesis scheme. Phase I: an amide bond is created at the C3 α-monoamino group of the anthracycline through the amine-reactive N-hydroxysuccinimide (NHS ester) group of succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC). Phase-II: the sulfhydryl-reactive maleimide group of the epirubicin-(C3-amide) reactive intermediate becomes covalently linked at/to thiolated lysine ε-amine groups residing within anti-HER2/neu monoclonal immunoglobulin fractions.
