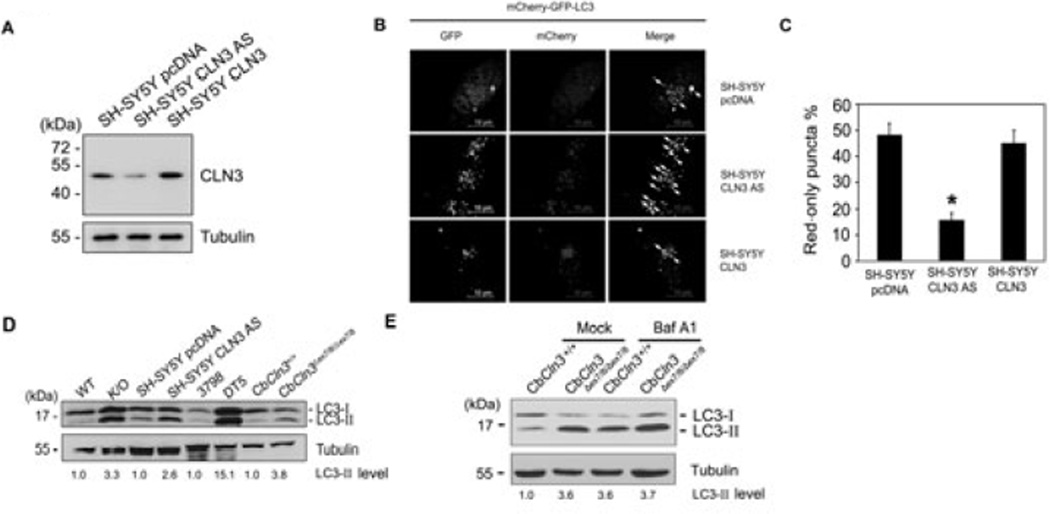
Fig. 1. Conversion of LC3-I to LC3-II is enhanced by CLN3 deficiency.
(A) Generation of CLN3 knockdown cells. SH-SY5Y cells were stably transfected with control pcDNA (SH-SY5Y pcDNA), pCLN3 AS (SH-SY5Y CLN3 AS; anti-sense cDNA of CLN3) or pCLN3 (SH-SY5Y CLN3), after which CLN3 expression was assessed by Western blotting with anti-CLN3 antibody. (B and C) Defective autophagic maturation in CLN3 knockdown cells. SH-SY5Y pcDNA, SH-SY5Y CLN3 or SH-SY5Y CLN3 AS cells were transiently transfected with mCherry-GFP-LC3 for 48 h and then examined under a confocal microscope. Arrows indicate cellular colocalization of mCherry and GFP (B). The numbers of cells showing only red fluorescence in B were quantified and are represented as bars (means ± S.D., n > 30 cells). Asterisk indicates a significant difference from control (p < 0.05) (C). (D) CLN3 deletion increases LC3-II levels. Whole brains extracts from Cln3 knock-out (K/O) mice and age-matched control (WT) mice, cell extracts from SH-SY5Y pcDNA and SH-SY5Y CLN3 AS cells, lymphoblastoid cell extracts of JNCL patients (DT5) and age-matched controls (3798), and cerebellar cell extracts from CbCln3+/+ (wild-type) and CbCln3Δex7/8/Δex7/8 (homozygous CbCln3Δex7/8) mice were analyzed by Western blotting using anti-LC3 and anti-Tubulin antibodies. LC3-II levels on the blot were quantified by densitometric analysis and normalized by those of Tubulin. Their relative ratios to the control (WT) are represented (LC3-II level). (E) Defective autophagic maturation in Cln3 knock-in cerebellar cells. CbCln3+/+ and CbCln3Δex7/8/Δex7/8 cerebellar cells were left untreated (Mock) or incubated with 20 nM bafilomycin A1 (Baf A1) for 2 h. Cell extracts were then prepared and analyzed by Western blotting using anti-LC3 antibody. The LC3-II signals on the blot were quantified as described in D.