Fig. 1.
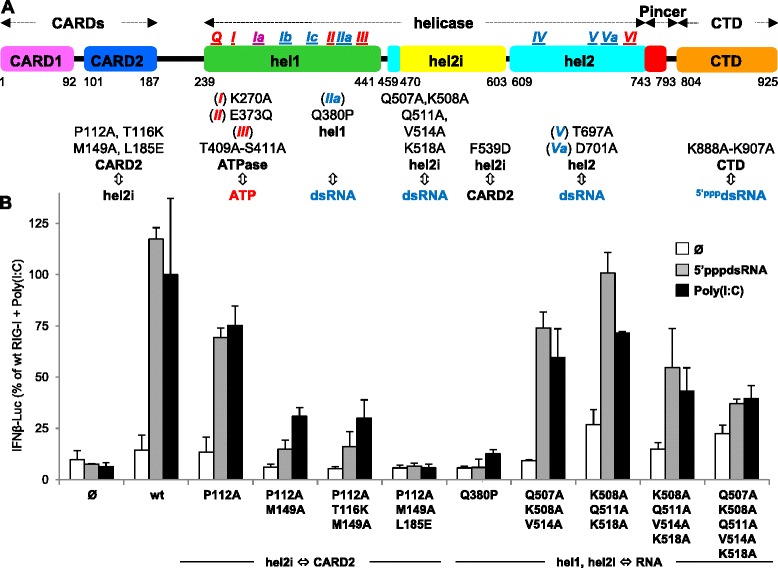
Refinement of RIG-I hel residues involved in hel2i-CARD2 interaction and RNA binding as assessed by RIG-I ability to activate the human IFNβ-promoter in Huh7.5 cells. a Modular domain organization of RIG-I and location of amino acid substitution investigated in this study. hel motifs involved in ATP binding or RNA binding are represented in blue and red, respectively, and in violet when involved in both of them. b Functional investigation in Huh7.5 cells of hel2i residues contacting CARD2 F539 residue in RIG-I auto-repressed form and hel1/hel2i residues involved in RNA binding without RNA (white columns), or stimulated with 5′pppdsRNA (grey columns) or poly(I:C) (black columns). Data (mean +/− SD of three independent replicates, and the experiment was performed twice with similar results) were expressed in fold response of wt RIG-I with poly(I:C) set up as 100 % activity. See Additional file 2 for protein expression levels of each construct analyzed by western blot. CARD: caspase activation and recruitment domain; hel: helicase
