Fig. 2.
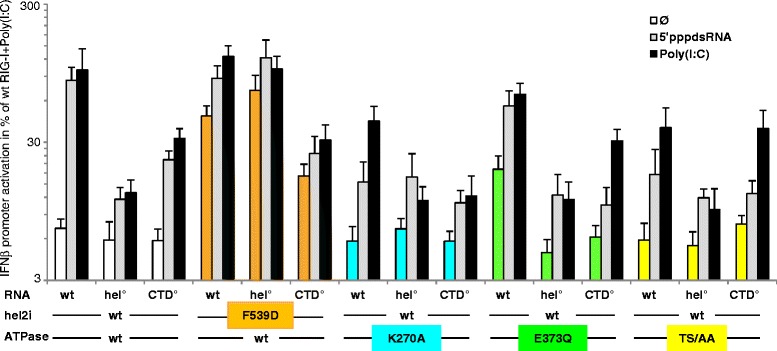
Interplay between the ATPase activity and RNA binding sites of RIG-I to elicit a signal. The ability of wt, hel2i F539D, ATPase E373Q, K270A and T409A/S411A (TS/AA) mutants RIG-I harboring or not mutations that loosen the RNA binding sites on the hel (T697A/E702A, hel°) or CTD (K888A/K907A, CTD°) domain were tested for their ability to activate the human IFNβ promoter. Note lower signalling ability of the F539D-CTD° mutant correlates with its lower expression as determined by western blot (see Additional file 2). The activity of F539D and E373Q was constitutive at P <0.0025 and P <0.01, respectively. All ATPase variants with intact hel and CTD domains significantly responded to both 5′pppdsRNA and poly(I:C) with the possible exception of TS/AA variant stimulated by the 5′pppdsRNA (P <0.05 to <0.005 range, in comparison with the signal without exogenous dsRNA). The wt, K270A, E373Q and TS/AA CTD° variants lost their response to 5′pppdsRNA (P <0.025 to <0.01 range in comparison with intact CTD counterparts), and also to the poly(I:C) in the case of K270A mutant (P <0.01). E373Q-hel° was inactive (P <0.001 versus 373Q) as were all hel° constructs (P <0.05 to <0.025 range versus counterparts with intact hel domain). The constitutive activity of the F539D mutant (P <0.0025 versus wt) remained unaffected when associated to either CTD° or hel° if the lower protein expression level of F539D-CTD° construct is taken into account. The significant response of F539D construct to both dsRNAs (P <0.05 and P <0.01 versus no RNA) was abolished if associated with hel° or CTD°. Note the log scale display of the IFNβ activation because values were within a large range. See Additional file 2 for protein expression levels of each construct analyzed by western blot. CTD: C-terminal domain; hel: helicase
