Fig. 3.
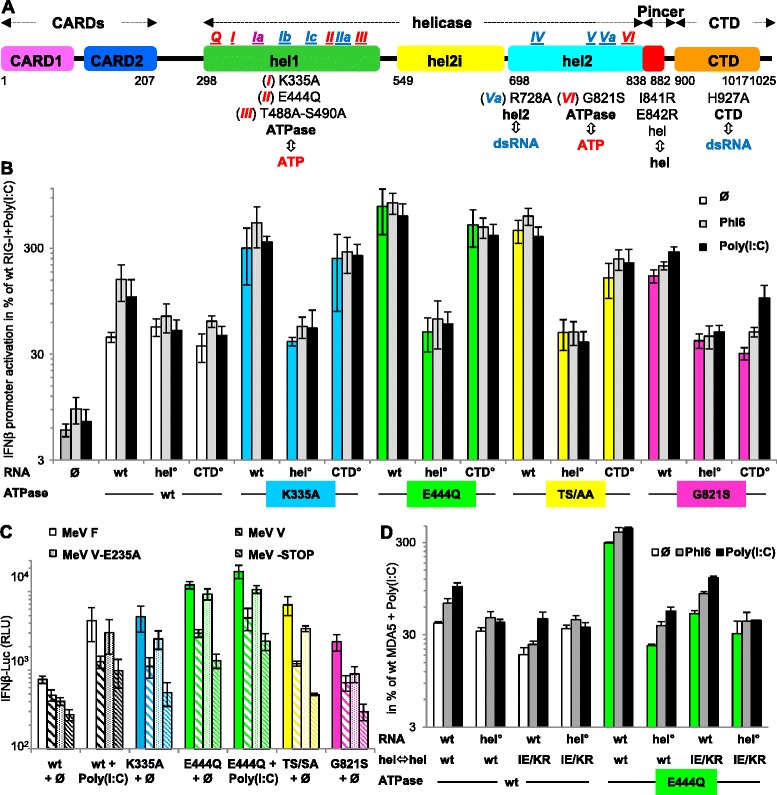
Interplay between the ATPase activity and RNA binding sites of MDA5 to elicit a signal. a Schematic domain organization of human MDA5. b Ability of MDA5 ATPase mutants to activate the IFNβ promoter in Huh7.5 cells. Expression of MDA5 activates the IFN promoter (P <0.0005). Compared to wt, ATPase variants with intact RNA binding sites exhibit a constitutive activity (P <0.05 to <0.0025). In contrast, the wt-CTD° and wt-hel° constructs, which have an altered RNA binding site due to R728A and H927A substitution, respectively, do not respond to both dsRNAs. The association of CTD° reduces the constitutive activity of only G821S (P <0.0025), with G821S-CTD° exhibiting a residual response to both dsRNAs (Phi6, P <0.025, poly(I:C), P <0.0025). Hel° inhibits both constitutive and RNA-dependent activities (P <0.05 to <0.005) down to the background activation level of IFNβ promoter observed with wt MDA5 (P <0.01 to <0.0025). c Inhibition of constitutive activity of every ATPase mutant by MeV V (P <0.025 to <0.0005 versus MeV F control) and MeV V-STOP (P <0.01 to <0.0005), but not by MeV V-E235A (P <0.05 to <0.0005). MeV V-STOP had higher inhibitory effect than MeV V (P <0.05 to <0.0005). In the presence of poly(I:C), the profile of inhibition of wt and E444Q MDA5 activity by MeV V and V-STOP was similar (MeV V or V-STOP versus F, P <0.05 to <0.005 and V-E235A versus V or V-STOP, P <0.05 to <0.0005). In panel (C), E444Q displayed the strongest constitutive activity (P <0.025 to <0.005), and K335A, TS/AA and G821S shared a similar constitutive activity. d IE/KR substitution alone significantly reduced observed responses of all constructs (P <0.05 to <0.0005 versus wt). As observed in panel (B) the introduction of hel° abolished both the response to dsRNA and constitutive activity of MDA5 variants (P <0.01 to <0.0005). Upon stimulation with Phi6 and poly(I:C), both wt and E444Q MDA5 with IE/KR substitution retained a significant activity (P <0.0025, P <0.01, P <0.0025 and P <0.0005, respectively), with a significantly higher response to poly(I:C) than to Phi6 (P <0.0125 and P <0.0025). See Additional file 2 for protein expression levels of each construct analyzed by western blot. CTD: C-terminal domain; hel: helicase; MeV: measles virus
