Fig. 4.
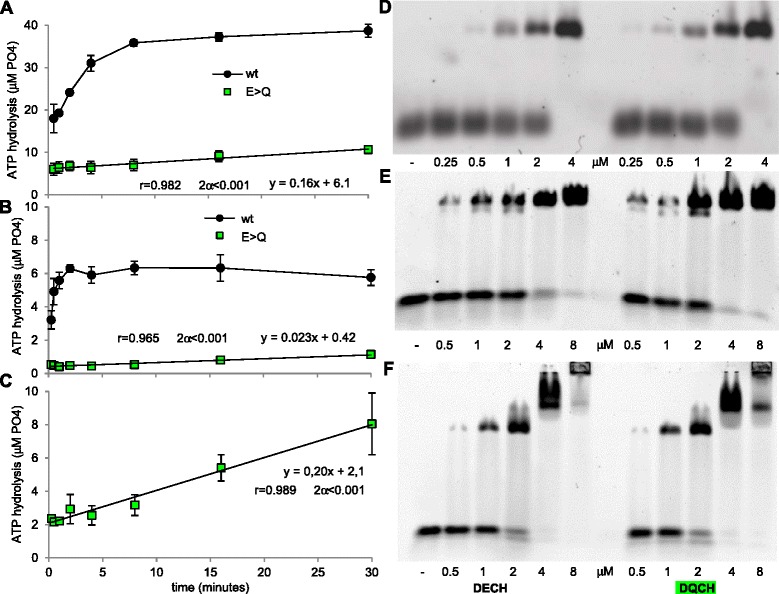
DECH/DQCH mutation in ATPase domain of a dRIG-I and b chMDA5 results in much slower ATP hydrolysis ability without affecting RNA binding and ablation of CARDs from chMDA5 DECH/DQCH mutant (hel-CTD construct), enhancing the c ATP hydrolysis rate. The hydrolysis of ATP by all three DECH/DQCH mutants increased linearly over 30 minutes as shown by equations and correlation analysis. ATP hydrolysis assay was performed by incubating a 0.5 μM RIG-I, 2 μM 12dsRNA, and b, c 2 mM ATP or 8 μM MDA5, 32 μM 24dsRNA, 2 mM ATP. d, e, f Representative EMSA showing the ability of wt (DECH) and DQCH proteins to bind to dsRNA. Each lane contains 40 ng cy3 labelled 12dsRNA (EMSA of duck RIG-I), 24dsRNA (EMSA of chicken MDA5), and increasing the concentration of d dRIG-I or e chMDA5 or f chMDA5 hel-CTD concentrations (0.25–8 μM). CARD: caspase activation and recruitment domain; CTD: C-terminal domain; EMSA: electrophoretic mobility shift assay; hel: helicase
