Fig.3.
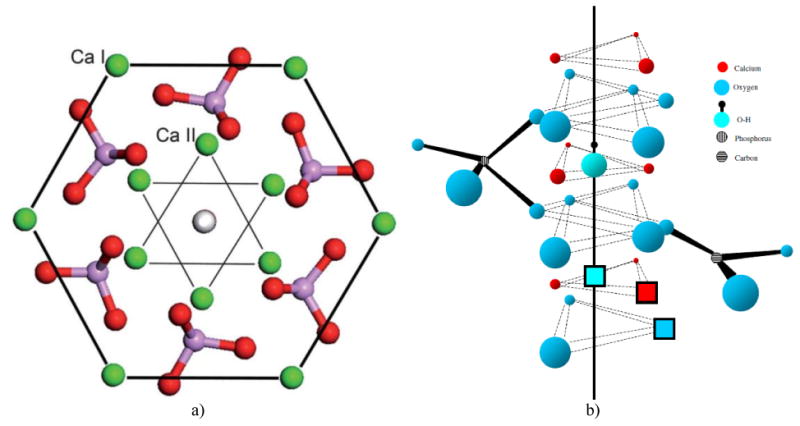
(a) A view of the hexagonal unit cell of HAp when projected down the c, [001] axis. Three parallel hexagons with columnar calcium atoms (Ca1) on their corners, positioned at the two basal planes (z = 0 and z = 1) and at the equatorial mirror plane in-between them (z = 1/2), define the geometric form of the unit cell, while hexagonal calcium atoms (Ca2) form a pair of inner triangles (z = 1/4 and z = 3/4) positioned at a 60° angle with respect to each other. Six PO4 tetrahedra occupying the interior of the unit cell are distributed in such a way so that three of them lie on the Ca2 triangle plane at z = 1/4 and three of them lie on the Ca2 triangle plane at z = ¾, themselves forming triangles at the 60° angle with respect to the in-plane Ca2 triangles and with respect to one another. Reprinted with permission from Ref.144. (b) The effect of phosphate-to-carbonate substitution on the creation of paired vacancies at adjacent Ca2 and hydroxyl group sites. The solid line represents the axis of the OH- channel formed by the series of overlapping calcium triangles and running perpendicular to the basal plane of the hexagonal unit cell. Reprinted with permission from Ref.145.
