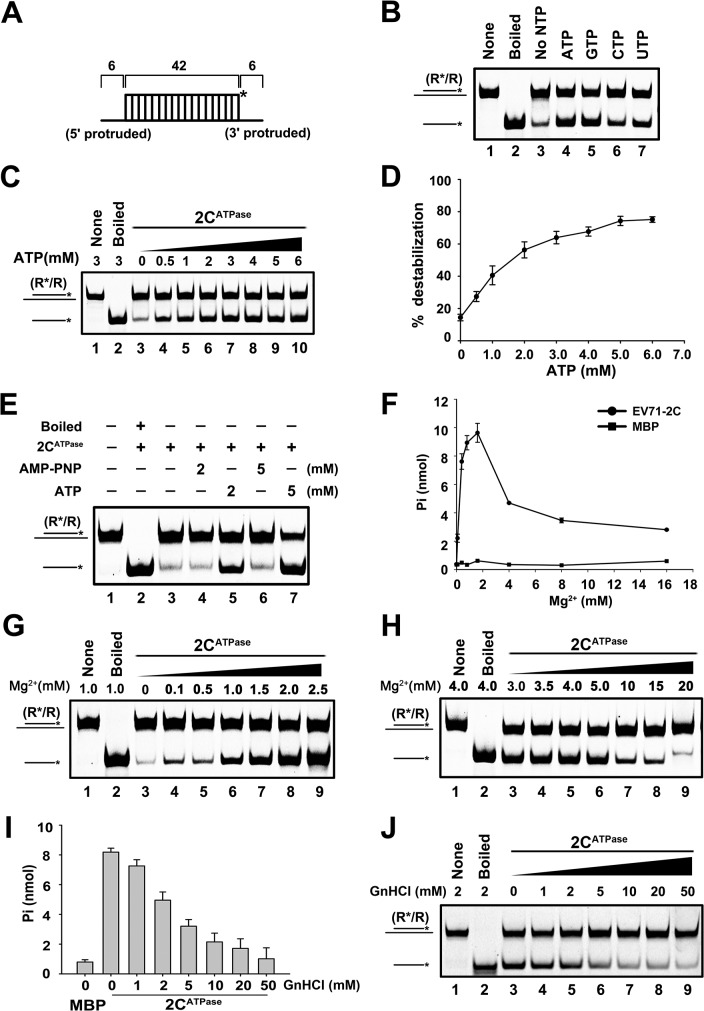
Fig 4. EV71 2CATPase possesses both ATP-dependent and ATP-independent helix unwinding activities.
(A) Schematic illustration of the standard RNA helix substrate that contains both 3′- and 5′-protrusions. (B) and (C) The standard RNA helix (0.1 pmol) was reacted with MBP-2CATPase in the absence or presence of the indicated NTPs (5 mM) (B) or in the presence of increasing concentrations (0–6 mM) of ATP as indicated (C). (D) The unwinding activities under different ATP concentrations were plotted as the percentage of the released RNA from the total RNA helix substrate (Y-axis) at each ATP concentration (X-axis). Error bars represent standard deviation (SD) values from three separate experiments. (E) Unwinding assays of 2CATPase using the standard helix substrate were performed in the absence or presence of ATP or ATP analog (AMP-PNP) as indicated. (F) The ATPase activity of MBP-2CATPase was measured as nanomoles of released inorganic phosphate at the indicated Mg2+ concentrations. Error bars represent SD values from three separate experiments. (G) and (H) Unwinding assays of 2CATPase were performed in the presence of 0–2.5 mM (G) or 3–20 mM (H) Mg2+ as indicated. (I) The ATPase activity of MBP-2CATPase was measured as nanomoles of released inorganic phosphate at the indicated GnHCl concentrations. Error bars represent SD values from three separate experiments. (J) Unwinding assays of 2CATPase were performed in the presence of 0–50 mM GnHCl as indicated.